Page 269 - Mechanism and Theory in Organic Chemistry
P. 269
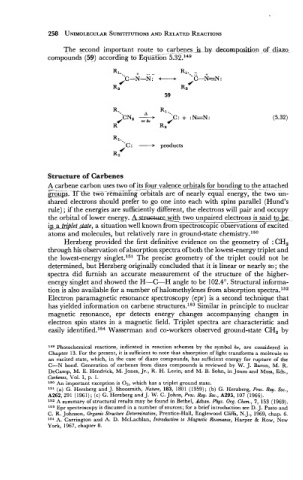
The second important route to carbenes js hy decomposition _of diazo-
compounds (59) according to EquSGn 5.32.149
'C: + products
4
R,
Structure of Carbenes
A carbene carbon uses two of its four-v~e_n~e_orbitals bonding to the attached
for
---
---
_.- ---
--- - -.
-
groups. Tf-The two-rEmaining orbitals are of nearly equal energy, the two un-
shared electrons should prefer to go one into each with spins parallel (Hund's
rule) ; if the energies are sufficiently different, the electrons will pair and occupy
the orbital of lower energy. w i t h two unpaired electrons --- is said toh
i~amlet &ate, a situation well known from spectroscopic observations of excited
atoms and molecules, but relatively rare in ground-state chemistry.150
Herzberg provided the first definitive evidence on the geometry of : CH,
through his observation of absorption spectra of both the lowest-energy triplet and
the lowest-energy singlet.151 The precise geometry of the triplet could not be
determined, but Herzberg originally concluded that it is linear or nearly so; the
spectra did furnish an accurate measurement of the structure of the higher-
energy singlet and showed the H-C--H angle to be 102.4". Structural informa-
tion is also available for a number of halomethylenes from absorption spectra.15,
Electron paramagnetic resonance spectroscopy (epr) is a second technique that
has yielded information on carbene structures.153 Similar in principle to nuclear
magnetic resonance, epr detects energy changes accompanying changes in
electron spin states in a magnetic field. Triplet spectra are characteristic and
easily identified.154 Wasserman and co-workers observed ground-state CH, by
148 Photochemical reactions, indicated in reaction schemes by the symbol hv, are considered in
Chapter 13. For the present, it is sufficient to note that absorption of light transforms a molecule to
an excited state, which, in the case of diazo compounds, has sufficient energy for rupture of the
C-N bond. Generation of carbenes from diazo compounds is reviewed by W. J. Baron, M. R.
Decamp, M. E. Hendrick, M. Jones, Jr., R. H. Levin, and M. B. Sohn, in Jones and Moss, Eds.,
Carbenes, Vol. I, p. 1.
160 An important exception is 02, which has a triplet ground state.
151 (a) G. Herzberg and J. Shoosmith, Nature, 183, 1801 (1959); (b) G. Herzberg, PTOC. Roy. SOC.,
A262, 291 (1961) ; (c) G. Herzberg and J. W. C. Johns, Proc. Roy. Sod., A295, 107 (1966).
152 A summary of structural results may be found in Bethel, Advan. Phys. Org. Chem., 7, 153 (1969).
153 Epr spectroscopy is discussed in a number of sources; for a brief introduction see D. J. Pasto and
C. R. Johnson, Organic Structure Determination, Prentice-Hall, Englewood Cliffs, N.J., 1969, chap. 6.
154 A. Carrington and A. D. McLachlan, Introduction to Magnetic Resonance, Harper & Row, New
York, 1967, chapter 8.