Page 264 - Mechanism and Theory in Organic Chemistry
P. 264
Unimolecular Electrophilic Substitutions-Carbanions 253
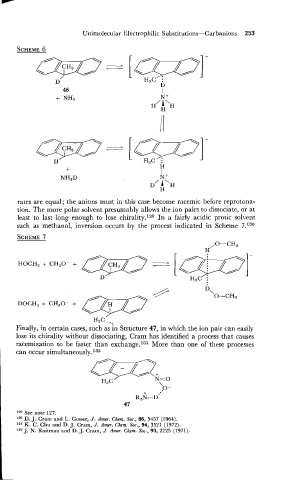
rates are equal; the anions must in this case become racemic before reprotona-
tion. The more polar solvent presumably allows the ion pairs to dissociate, or at
least to last long enough to lose chirality.129 In a fairly acidic protic solvent
such as methanol, inversion occurs by the process indicated in Scheme 7.130
,
H" ,
Finally, in certain cases, such as in Structure 47, in which the ion pair can easily
lose its chirality without dissociating, Cram has identified a process that causes
racemization to be faster than exchange.131 More than one of these processes
can occur simultaneously.132
3-
+ :
K3N-D
47
lZ8 See note 127.
D. J. Cram and L. Gosser, J. Amer. Chem. SOL, 86, 5457 (1964).
131 K. C. Chu and D. J. Cram, J. Amer. Chem. SOC., 94, 3521 (1972).
132 J. N. Roitman and D. J. Cram, J. Amer. Chem. SOC., 93, 2225 (1971).