Page 261 - Mechanism and Theory in Organic Chemistry
P. 261
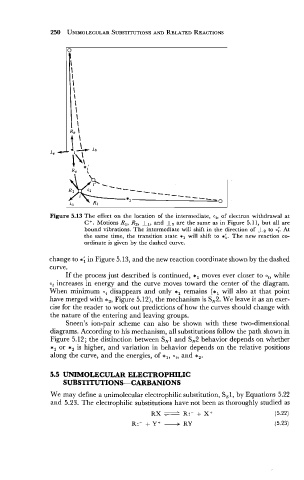
Figure 5.13 The effect on the location of the intermediate, o,, of electron withdrawal at
C+. Motions R1, R2, I,, and I2 are the same as in Figure 5.1 1, but all are
bound vibrations. The intermediate will shift in the direction of 1, to 0;. At
the same time, the transition state *, will shift to *;. The new reaction co-
ordinate is given by the dashed curve.
change to *; in Figure 5.13, and the new reaction coordinate shown by the dashed
curve.
If the process just described is continued, *, moves ever closer to oi, while
0, increases in energy and the curve moves toward the center of the diagram.
When minimum oi disappears and only *, remains (*, will also at that point
have merged with *,, Figure 5.12), the mechanism is SN2. We leave it as an exer-
cise for the reader to work out predictions of how the curves should change with
the nature of the entering and leaving groups.
Sneen's ion-pair scheme can also be shown with these two-dimensional
diagrams. According to his mechanism, all substitutions follow the path shown in
Figure 5.12; the distinction between SN1 and SN2 behavior depends on whether
*, or *, is higher, and variation in behavior depends on the relative positions
along the curve, and the energies, of *,, o,, and *,.
5.5 UNIMOLECULAR ELECTROPHILIC
SUBSTITUTIONS-CARBANIONS
We may define a unimolecular electrophilic substitution, S,1, by Equations 5.22
and 5.23. The electrophilic substitutions have not been as thoroughly studied as