Page 265 - Mechanism and Theory in Organic Chemistry
P. 265
Anions stabilized by adjacent carbonyl groups (44) usually give racemiza-
tion no matter what the solvent. The charge is distributed between carbon and
oxygen; the hard proton acid prefers the hard end of the ambident base and adds
to the 0~ygen.l~~ An en01 results, and it lasts long enough before changing to
the more stable keto form for its environment to become symmetric.134 Cyclo-
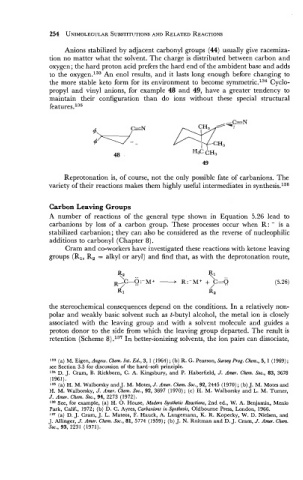
propyl and vinyl anions, for example 48 and 49, have a greater tendency to
maintain their configuration than do ions without these special structural
features.135
Reprotonation is, of course, not the only possible fate 'of carbanions. The
variety of their reactions makes them highly useful intermediates in synthesis.13"
Carbon Leaving Groups
A number of reactions of the general type shown in Equation 5.26 lead to
carbanions by loss of a carbon group. These processes occur when R: - is a
stabilized carbanion; they can also be considered as the reverse of nucleophilic
additions to carbonyl (Chapter 8).
Cram and co-workers have investigated these reactions with ketone leaving
groups (R,, R, = alkyl or aryl) and find that, as with the deprotonation route,
the stereochemical consequences depend on the conditions. In a relatively non-
polar and weakly basic solvent such as t-butyl alcohol, the metal ion is closely
associated with the leaving group and with a solvent molecule and guides a
proton donor to the side from which the leaving group departed. The result is
retention (Scheme 8) .I3' In better-ionizing solvents, the ion pairs can dissociate,
133 (a) M. Eigen, Angew. Chem. Int. Ed., 3, 1 (1964); (b) R. G. Pearson, Survey Prog. Chem., 5, 1 (1969);
see Section 3.5 for discussion of the hard-soft principle.
134 D. J. Cram, B. Rickborn, C. A. Kingsbury, and P. Haberfield, J. Amer. Chem. SOC., 83, 3678
(1961).
(a) H. M. Walborsky and J. M. Motes, J. Amer. Chem. Soc., 92, 2445 (1970); (b) J. M. Motes and
H. M. Walborsky, J. Amer. Chem. Soc., 92, 3697 (1970); (c) H. M. Walborsky and L. M. Turner,
J. Amer. Chem. Soc., 94, 2273 (1972).
136 See, for example, (a) H. 0. House, Modern Synthetic Reactions, 2nd ed., W. A. Benjamin, Menlo
Park, Calif., 1972; (b) D. C. Ayres, Carbanions in Synthesis, Oldbourne Press, London, 1966.
137 (a) D. J. Cram, J. L. Mateos, F. Hauck, A. Langemann, K. R. Kopecky, W. D. Nielsen, and
J. Allinger, J. Amer. Chem. Soc., 81, 5774 (1959); (b) J. N. Roitman and D. J. Cram, J. Amr. Chem.
SOC., 93, 2231 (1971).