Page 267 - Mechanism and Theory in Organic Chemistry
P. 267
5.6 CARBENES
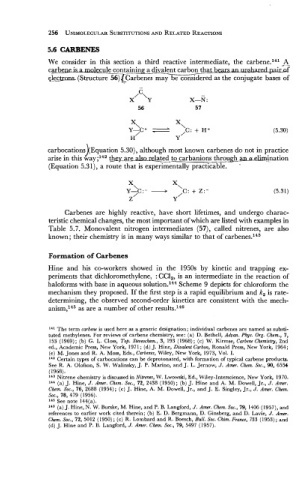
We consider in this section a third reactive intermediate, the carbene.141 -A-x
--
---
carbe ' cule containing a divalent carbon that be- us
e - - e ! c 5q~arbenes may be considered as the conjum
C
/ \
X Y X-N :
56 57
J
carbocations Equation 5.30), although most known carbenes do not in practice
arise in this way;14, tby are also aed to carbanions --- throu&-cr-&.ination
(Equation 5.3 1) , a route that is experimentally practicable. -
Carbenes are highly reactive, have short lifetimes, and undergo charac-
teristic chemical changes, the most important of which are listed with examples in
Table 5.7, Monovalent nitrogen intermediates (57), called nitrenes, are also
known; their chemistry is in many ways similar to that of ~arbenes.~~~
Formation of Carbenes
Hine and his co-workers showed in the 1950s by kinetic and trapping ex-
periments that dichloromethylene, : CCl,, is an intermediate in the reaction of
haloforms with base in aqueous s01ution.l~~ Scheme 9 depicts for chloroform the
mechanism they proposed. If the first step is a rapid equilibrium and k, is rate-
determining, the observed second-order kinetics are consistent with the mech-
ani~m,l~~ are a number of other re~u1ts.l~~
as
'41 The term carbene is used here as a generic designation; individual carbenes are named as substi-
tuted methylenes. For reviews of carbene chemistry, see: (a) D. Bethell, Advan. Phys. Org. Chem., 7,
153 (1969); (b) G. L. Closs, TOP. Stereochem., 3, 193 (1968); (c) W. Kirmse, Carbene Chemistry, 2nd
ed., Academic Press, New York, 1971 ; (d) J. Hine, Divabnt Carbon, Ronald Press, New York, 1964;
(e) M. Jones and R. A. Moss, Eds., Carbenes, Wiley, New York, 1973, Vol. I.
142 Certain types of carbocations can be deprotonated, with formation of typical carbene products.
See R. A. Olofson, S. W. Walinsky, J. P. Marino, and J. L. Jernow, J. Amer. Chem. SOC., 90, 6554
(1968).
143 Nitrene chemistry is discussed in Nitrenes, W. Lwowski, Ed., Wiley-Interscience, New York, 1970.
144 (a) J. Hine, J. Amer. Chem. Soc., 72, 2438 (1950); (b) J. Hine and A. M. Dowell, Jr., J. Amer.
Chem. SOC., 76, 2688 (1954); (c) J. Hine, A. M. Dowell, Jr., and J. E. Singley, Jr., J. Amer. Chm.
SOC., 78, 479 (1956).
145 See note 144(a).
14@ (a) J. Hine, N. W. Burske, M. Hine, and P. B. Langford, J. Amer. Chem. Soc., 79, 1406 (1957), and
references to earlier work cited therein; (b) E. D. Bergmann, D. Ginsberg, and D. Lavie, J. Amcr.
Chem. SOC., 72, 5012 (1950); (c) R. Lombard and R. Boesch, Bull. Soc. Chim. France, 733 (1953); and
(d) J. Hine and P. B. Langford, J. Amer. Chem. SOC., 79, 5497 (1957).