Page 266 - Mechanism and Theory in Organic Chemistry
P. 266
Unimolecular Electrophilic Substitutions-Carbanions 255
and racemization results if the solvent is aprotic and inversion if the solvent can
donate a proton from the back side.13B
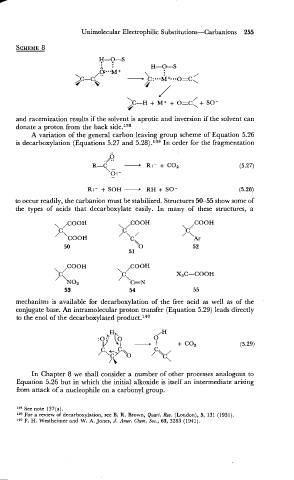
A variation of the general carbon leaving group scheme of Equation 5.26
is decarboxylation (Equations 5.27 and 5.28).139 In order for the fragmentation
RH
R:- + SOH - + SO- (5.28)
to occur readily, the carbanion must be stabilized. Structures 5C55 show some of
the types of acids that decarboxylate easily. In many of these structures, a
mechanism is available for decarboxylation of the free acid as well as of the
conjugate base. An intramolecular proton transfer (Equation 5.29) leads directly
to the en01 of the decarboxylated product.140
In Chapter 8 we shall consider a number of other processes analogous to
Equation 5.26 but in which the initial alkoxide is itself an intermediate arising
from attack of a nucleophile on a carbonyl group.
138 See note 137(a).
130 For a review of decarboxylation, see B. R. Brown, Quart. Rev. (London), 5, 13 1 (1951).
140 F. H. Westheimer and W. A. Jones, J. Amer. Chem. Sac., 63, 3283 (1941).