Page 260 - Mechanism and Theory in Organic Chemistry
P. 260
Mechanisms Intermediate Between SN1 and SN2 249
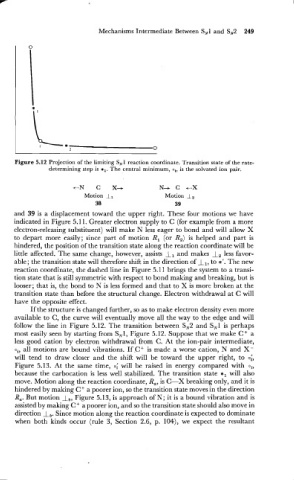
Figure 5.12 Projection of the limiting SN1 reaction coordinate. Transition state of the rate-
determining step is *,. The central minimum, o,, is the solvated ion pair.
+-N C X+ N+ C c-X
Motion 1, Motion I,
38 39
and 39 is a displacement toward the upper right. These four motions we have
indicated in Figure 5.1 1. Greater electron supply to C (for example from a more
electron-releasing substituent) will make N less eager to bond and will allow X
to depart more easily; since part of motion R, (or R,) is helped and part is
hindered, the position of the transition state along the reaction coordinate will be
little affected. The same change, however, assists 1, and makes 1, less favor-
able; the transition state will therefore shift in the direction of I,, to *'. The new
reaction coordinate, the dashed line in Figure 5.11 brings the system to a transi-
tion state that is still symmetric with respect to bond making and breaking, but is
looser; that is, the bond to N is less formed and that to X is more broken at the
transition state than before the structural change. Electron withdrawal at C will
have the opposite effect.
If the structure is changed further, so as to make electron density even more
available to C, the curve will eventually move all the way to the edge and will
follow the line in Figure 5.12. The transition between SN2 and SN1 is perhaps
most easily seen by starting from SN1, Figure 5.12. Suppose that we make C+ a
less good cation by electron withdrawal from C. At the ion-pair intermediate,
oi, all motions are bound vibrations. If .C+ is made a worse cation, N and X-
will tend to draw closer and the shift will be toward the upper right, to o;,
Figure 5.13. At the same time, 0; will be raised in energy compared with oi,
because the carbocation is less well stabilized. The transition state *, will also
move. Motion along the reaction coordinate, R,, is C-X breaking only, and it is
hindered by making C+ a poorer ion, so the transition state moves in the direction
R,. But motion I,, Figure 5.13, is approach of N; it is a bound vibration and is
assisted by making C+ a poorer ion, and so the transition state should also move in
direction 1,. Since motion along the reaction coordinate is expected to dominate
when both kinds occur (rule 3, Section 2.6, p. 104), we expect the resultant