Page 275 - Mechanism and Theory in Organic Chemistry
P. 275
Carbenes 263
alkyl groups in the olefin, demonstrate that the methylenes and carbenoids are in
practice strong electrophiles. Reactivity of carbenoids is lower with the highly
substituted olefins, a result of steric hindrance considered to be evidence that
they are not free ~arbenes.l~~
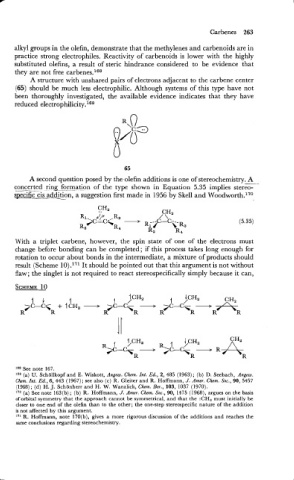
A structure with unshared pairs of electrons adjacent to the carbene center
(65) should be much less electrophilic. Although systems of this type have not
been thoroughly investigated, the available evidence indicates that they have
reduced ele~trophi1icity.l~~
A
A second question posed by the olefin additions is one of stereochemistry. -
--
concerted ring formation of the type shown in Equation 5.35 implies stereo-
specific cis adgtkn, a suggestion first made in 1956 by Skell and Woodw~rth.~~~
With a triplet carbene, however, the spin state of one of the electrons must
change before bonding can be completed; if this process takes long enough for
rotation to occur about bonds in the intermediate, a mixture of products should
result (Scheme 10).171 It should be pointed out that this argument is not without
flaw; the singlet is not required to react stereospecifically simply because it can,
, 4 CH,
1 1 1 p 2 1 /
;C-C- + 1 CH, + ;C-C- + ;C-C- +
R R ' R R ' R R . R
lee See note 167.
leg (a) U. Schollkopf and E. Wiskott, Angew. Chem. Znt. Ed., 2, 485 (1963) ; (b) D. Seebach, Angew.
Chem. Znt. Ed., 6, 443 (1967) ; see also (c) R. Gleiter and R. Hoffmann, J. Amer. Chem. Sac., 90, 5457
(1968); (d) H. J. Schonherr and H. W. Wanzlick, Chem. Ber., 103, 1037 (1970).
(a) See note 163(b); (b) R. Hoffmann, J. Amer. Chem. Sac., 90, 1475 (1968), argues on the basis
of orbital symmetry that the approach cannot be symmetrical, and that the :CH, must initially be
closer to one end of the olefin than to the other; the one-step stereospecific nature of the addition
is not affected by this argument.
lT1 R. Hoffmann, note 170(b), gives a more rigorous discussion of the additions and reaches the
same conclusions regarding stereochemistry.