Page 346 - Mechanism and Theory in Organic Chemistry
P. 346
Problems 333
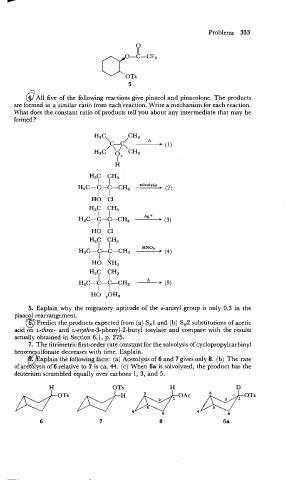
V..OTS
5
&? All five of the following reactions give pinacol and pinacolone. The products
are formed in a similar ratio from each reaction. Write a mechanism for each reaction.
What does the constant ratio of products tell you about any intermediate that may be
formed ?
\c-c" -
H3C CH3 A (I)
p+
/ ' ' \CH,
3
HO C1
H3C CH,
I I
H3C-C-C-CH3 Ag + > (3)
I I
5. Explain why the migratory aptitude of the o-anisyl group is only 0.3 in the
pinacol rearrangement.
wedict the products expected from (a) SNl and (b) SN2 substitutions of acetic
acid on L-threo- and L-erythro-3-phenyl-2-butyl tosylate and compare with the results
actually obtained in Section 6.1, p. 275.
7. The titrimetric first-order rate constant for the solvolysis of cyclopropylcarbinyl
benzen ulfonate decreases with time. Explain.
xplain the following facts: (a) Acetolysis of 6 and 7 gives only 8. (b) The rate
of ace olysis of 6
f9 relative to 7 is ca. 44. (c) When 6a is solvolyzed, the product has the
deuterium scrambled equally over carbons 1, 3, and 5.