Page 344 - Mechanism and Theory in Organic Chemistry
P. 344
Rearrangements to Electron-deficient Nitrogen and Oxygen 331
p=-1.45
-
1.8
5 + log k3
1.6 -
1.4 -
-
1.2
-0.2 0.0 0.2 0.4 0.6 0.8
a
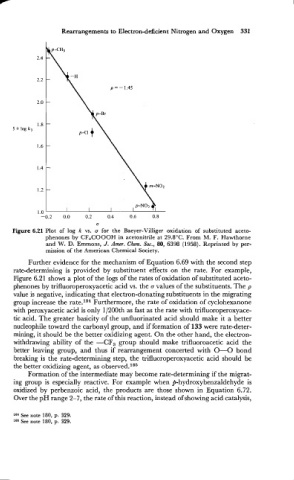
Figure 6.21 Plot of log k vs. u for the Baeyer-Villiger oxidation of substituted aceto-
phenones by CF,COOOH in acetonitrile at 29.8"C. From M. F. Hawthorne
and W. D. Emmons, J. Amer. Chem. Soc., 80, 6398 (1958). Reprinted by per-
mission of the American Chemical Society.
Further evidence for the mechanism of Equation 6.69 with the second step
rate-determining is provided by substituent effects on the rate. For example,
Figure 6.2 1 shows a plot of the logs of the rates of oxidation of substituted aceto-
phenones by trifluoroperoxyacetic acid vs. the a values of the substituents. The p
value is negative, indicating that electron-donating substituents in the migrating
group increase the rate.le4 Furthermore, the rate of oxidation of cyclohexanone
with peroxyacetic acid is only 11200th as fast as the rate with trifluoroperoxyace-
tic acid. The greater basicity of the unfluorinated acid should make it a better
nucleophile toward the carbonyl group, and if formation of 133 were rate-deter-
mining, it should be the better oxidizing agent. On the other hand, the electron-
withdrawing ability of the -CF, group should make trifluoroacetic acid the
better leaving group, and thus if rearrangement concerted with 0-0 bond
breaking is the rate-determining step, the trifluoroperoxyacetic acid should be
the better oxidizing agent, as observed.le5
Formation of the intermediate may become rate-determining if the migrat-
ing group is especially reactive. For example when p-hydroxybenzaldehyde is
oxidized by perbenzoic acid, the products are those shown in Equation 6.72.
Over the pH range 2-7, the rate of this reaction, instead of showing acid catalysis,
le4 See note 180, p. 329.
le6 See note 180, p. 329.