Page 343 - Mechanism and Theory in Organic Chemistry
P. 343
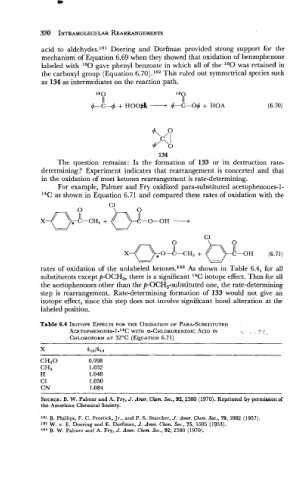
acid to aldehydes.lsl Doering and Dorfman provided strong support for the
mechanism of Equation 6.69 when they showed that oxidation of benzophenone
labeled with 180 gave phenyl benzoate in which all of the ''0 was retained in
the carboxyl group (Equation 6.70).ls2 This ruled out symmetrical species such
as 134 as intermediates on the reaction path.
IS 0 l80
II II
4-C-+ + HOO& - 4-C-04 + HOA (6.70)
134
The question remains: Is the formation of 133 or its destruction rate-
determining? Experiment indicates that rearrangement is concerted and that
in the oxidation of most ketones rearrangement is rate-determining.
For example, Palmer and Fry oxidized para-substituted acetophenones-l-
14C as shown in Equation 6.71 and compared these rates of oxidation with the
rates of oxidation of the unlabeled ketones.ls3 As shown in Table 6.4, for all
substituents except P-OCH,, there is a significant 14C isotope effect. Thus for all
the acetophenones other than the p-OCH3-substituted one, the rate-determining
step is rearrangement. Rate-determining formation of 133 would not give an
isotope effect, since this step does not involve significant bond alteration at the
labeled position.
Table 6.4 ISOTOPE EFFECTS FOR THE OXIDATION PARA-SUBSTITUTED
OF
ACETOPHENONES-~-~~C m-CHLOROBENZOIC ACID IN I) z #?If.,
WITH
CHLOROFORM AT 32OC (EQUATION 6.71)
x k12/k14
SOURCE: B. W. Palmer and A. Fry, J. Amer. Chem. Soc., 92, 2580 (1970). Reprinted by permission of
the American Chemical Society.
lB1 B. Phillips, F. C. Frostick, Jr., and P. S. Starcher, J. Amer. Chem. Soc., 79. 5982 (1957).
W. V. E. Doering and E. Dorfman, J. Amer. Chem. Soc., 75, 5595 (1953).
lB3 B. W. Palmer and A. Fry, J. Amer. Chem. Soc., 92, 2580 (1970).