Page 338 - Mechanism and Theory in Organic Chemistry
P. 338
Rearrangements to Electron-deficient Nitrogen and Oxygen 325
to determine whether or not it exists. Since nitrogen is more electronegative than
carbon, it was to be expected that the nitrenium ion would be less stable than its
carbon analog.
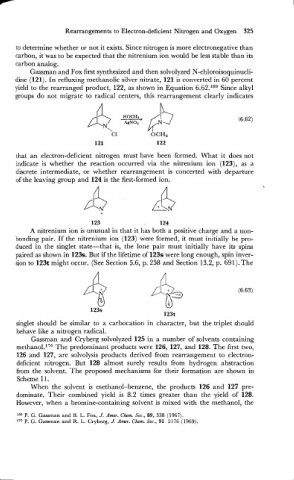
Gassman and Fox first synthesized and then solvolyzed N-chloroisoquinucli-
dine (121). In refluxing methanolic silver nitrate, 121 is converted in 60 percent
yield to the rearranged product, 122, as shown in Equation 6.62.169 Since alkyl
groups do not migrate to radical centers, this rearrangement clearly indicates
that an electron-deficient nitrogen must have been formed. What it does not
indicate is whether the reaction occurred via the nitrenium ion (123), as a
discrete intermediate, or whether rearrangement is concerted with departure
of the leaving group and 124 is the first-formed ion.
123 . 124
A nitrenium ion is unusual in that it has both a positive charge and a non-
bonding pair. If the nitrenium ion (123) were formed, it must initially be pro-
duced in the singlet state-that is, the lone pair must initially have its spins
paired as shown in 123s. But if the lifetime of 123s were long enough, spin inver-
sion to 123t might occur. (See Section 5.6, p. 258 and Section 13.2, p. 691).The
singlet should be similar to a carbocation in character, but the triplet should
behave like a nitrogen radical.
Gassman and Cryberg solvolyzed 125 in a number of solvents containing
methanol.170 The predominant products were 126, 127, and 128. The first two,
126 and 127, are solvolysis products derived from rearrangement to electron-
deficient nitrogen. But 128 almost surely results from hydrogen abstraction
from the solvent. The proposed mechanisms for their formation are shown in
Scheme 1 1.
When the solvent is methanol-benzene, the products 126 and 127 pre-
dominate. Their combined yield is 8.2 times greater than the yield of 128.
However, when a bromine-containing solvent is mixed with the methanol, the
16@ P. G. Gassman and B. L. Fox, J. Amer. Chm. Sac., 89, 338 (1967).
170 P. G. Gassman and R. L. Cryberg, J. Amr. Chem. Sac., 91 5176 (1969).