Page 340 - Mechanism and Theory in Organic Chemistry
P. 340
Rearrangements to Electron-deficient Nitrogen and Oxygen 327
Heterolytic Peroxyester Decomposition: The Criegee Rearrangement
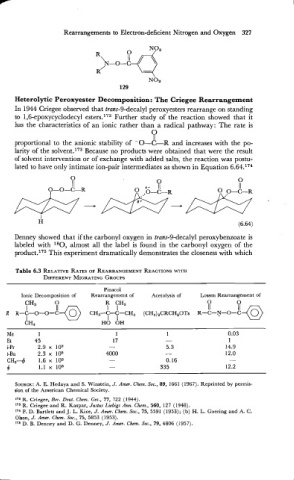
In 1944 Criegee observed that trans-9-decalyl peroxyesters rearrange on standing
to 1,6-epoxycyclodecyl esters.172 Further study of the reaction showed that it
has the characteristics of an ionic rather than a radical pathway: The rate is
8
proportional to the anionic stability of -0-C-R and increases with the po-
larity of the s01vent.l~~ Because no products were obtained that were the result
of solvent intervention or of exchange with added salts, the reaction was postu-
lated to have only intimate ion-pair intermediates as shown in Equation 6.64.174
Denney showed that if the carbonyl oxygen in trans-9-decalyl peroxybenzoate is
labeled with 180, almost all the label is found in the carbonyl oxygen of the
pr0d~ct.l~~ This experiment dramatically demonstrates the closeness with which
Table 6.3 RELATIVE RATES OF REARRANGEMENT ~ZEACTIONS WITH
DIFFERENT MIGRATING GROUPS
Pinacol
Ionic Decomposition of Rearrangement of Acetolysis of Lossen Rearrangement of
CH3 0 R CH, 0
II
I I I (CH,),,:RCH,OTS R-C-yo-la
.-.-.-.-e-@ I cH,c-c-cH,
I I
CH3 HO OH
SOURCE: E. Hedaya and S. Winstein, J. Amer. Chem. Sac., 89, 1661 (1967). Reprinted by permis-
A.
sion of the American Chemical Society.
R. Criegee, Ber. Deut. Chem. Ges., 77, 722 (1944).
R. Criegee and R. Kaspar, Justus Liebigs Ann. Chem., 560, 127 (1948).
P. D. Bartlett and J. L. Kice, J. Amer. Chem. Sac., 75, 5591 (1953); (b) H. L. Goering and A. C.
Olson, J. Amer. Chem. Soc., 75, 5853 (1953).
D. B. Denney and D. G. Denney, J. Amer. Chem. Sac., 79, 4806 (1957).