Page 337 - Mechanism and Theory in Organic Chemistry
P. 337
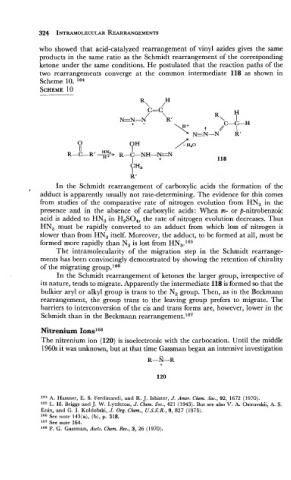
who showed that acid-catalyzed rearrangement of vinyl azides gives the same
products in the same ratio as the Schmidt rearrangement of the corresponding
ketone under the same conditions. He postulated that the reaction paths of the
two rearrangements converge at the common intermediate 118 as shown in
Scheme 10. 164
SCHEME 10
In the Schmidt rearrangement of carboxylic acids the formation of the
adduct is apparently usually not rate-determining. The evidence for this comes
from studies of the comparative rate of nitrogen evolution from HN, in the
presence and in the absence of carboxylic acids: When m- or p-nitrobenzoic
acid is added to HN, in H2S04, the rate of nitrogen evolution decreases. Thus
HN, must be rapidly converted to an adduct from which loss of nitrogen is
slower than from HN, itself. Moreover, the adduct, to be formed at all, must be
formed more rapidly than N, is lost from HN3.165
The intramolecularity of the migration step in the Schmidt rearrange-
ments has been convincingly demonstrated by showing the retention of chirality
of the migrating gr0~p.l~~
In the Schmidt rearrangement of ketones the larger group, irrespective of
its nature, tends to migrate. Apparently the intermediate 118 is formed so that the
bulkier aryl or alkyl group is trans to the N, group. Then, as in the Beckmann
rearrangement, the group trans to the leaving group prefers to migrate. The
barriers to interconversion of the cis and trans forms are, however, lower in the
Schmidt than in the Beckmann rearrangement.167
The nitrenium ion (120) is isoelectronic with the carbocation. Until the middle
1960s it was unknown, but at that time Gassman began an intensive investigation
R-N-R
+
'64 A. Hassner, E. S. Ferdinandi, and R. J. Isbister, J. Amer. Chem. Sac., 92, 1672 (1970).
'65 L. H. Briggs and J. W. Lyttleton, J. Chem. Sac., 421 (1943). But see also V. k Ostrovskii, A. S.
Enin, and G. I. Koldobski, J. 018. Chem., U.S.S.R., 9, 827 (1973).
See note 143(a), (b), p. 318.
See note 164.
168 P. G. Gassman, Accts. Chem. Res., 3, 26 (1970).