Page 334 - Mechanism and Theory in Organic Chemistry
P. 334
Rearrangements to Electron-deficient Nitrogen and Oxygen 321
-
I
6 + log k A m-C1 and m-Br
-
p=-1.1
-
p-NOz A
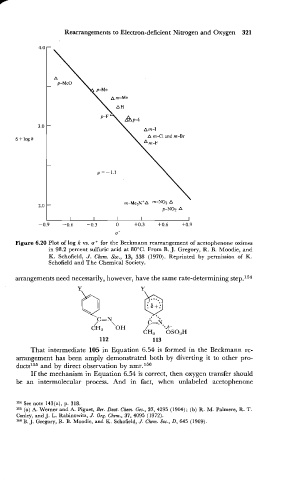
Figure 6.20 Plot of log k vs. a+ for the Beckmann rearrangement of acetophenone oximes
in 98.2 percent sulfuric acid at 80°C. From B. J. Gregory, R. B. Moodie, and
K. Schofield, J. Chem. Soc., 13, 338 (1970). Reprinted by permission of K.
Schofield and The Chemical Society.
arrangements need necessarily, however, have the same rate-determining step.154
That intermediate 105 in Equation 6.54 is formed in the Beckmann re-
arrangement has been amply demonstrated both by diverting it to other pro-
duct~~~~ direct observation by nmr.156
by
and
If the mechanism in Equation 6.54 is correct, then oxygen transfer should
be an intermolecular process. And in fact, when unlabeled acetophenone
154 See note 143(a), p. 318.
155 (a) A. Werner and A. Piguet, Ber. Deut. Chem. Ges., 37, 4295 (1904); (b) R. M. Palmere, R. T.
Conley, and J. L. Rabinowitz, J. Org. Chem., 37, 4095 (1972).
15e B. J. Gregory, R. B. Moodie, and K. Schofield, J. Chem. Soc., D, 645 (1969).