Page 329 - Mechanism and Theory in Organic Chemistry
P. 329
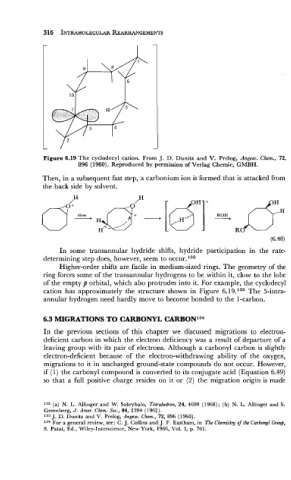
Figure 6.19 The cyclodecyl~cation. From J. D. Dunitz and V. Prelog, An,eew. Chem., 72,
896 (1960). Reproduced by permission of Verlag Chemie, GMBH.
Then, in a subsequent fast step, a carbonium ion is formed that is attacked from
the back side by solvent.
In some transannular hydride shifts, hydride participation in the rate-
determining step does, however, seem to occur.132
Higher-order shifts are facile in medium-sized rings. The geometry of the
ring forces some of the transannular hydrogens to be within it, close to the lobe
of the empty p orbital, which also protrudes into it. For example, the cyclodecyl
cation has approximately the structure shown in Figure 6.19.133 The 5-intra-
annular hydrogen need hardly move to become bonded to the 1-carbon.
6.3 MIGRATIONS TO CARBONYL CARBON134
In the previous sections of this chapter we discussed migrations to electron-
deficient carbon in which the electron deficiency was a result of departure of a
leaving group with its pair of electrons. Although a carbonyl carbon is slightly
electron-deficient because of the electron-withdrawing ability of the oxygen,
migrations to it in uncharged ground-state compounds do not occur. However,
if (1) the carbonyl compound is converted to its conjugate acid (Equation 6.49)
so that a full positive charge resides on it or (2) the migration origin is made
13= (a) N. L. Allinger and W. Szkrybalo, Tetrahedron, 24, 4699 (1968); (b) N. L. Allinger and S.
Greenberg, J. Amer. Chem. SOG., 84, 2394 ( 1962).
J. D. Dunitz and V. Prelog, Angew. Chem., 72, 896 (1960).
134 For a general review, see: C. J. Collins and J. F. Eastham, in The Chemistry of the Carbonyl Group,
S. Patai, Ed., Wiley-Interscience, New York, 1966, Vol. I, p. 761.