Page 325 - Mechanism and Theory in Organic Chemistry
P. 325
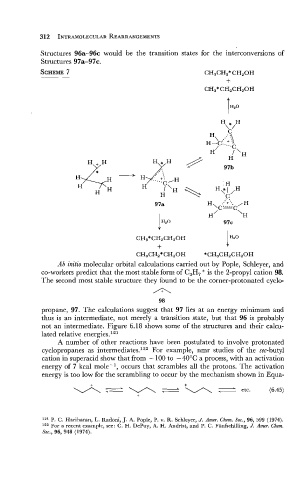
Structures 96a-96c would be the transition states for the interconversions of
Structures 97a-97c.
Ab initio molecular orbital calculations carried out by Pople, Schleyer, and
co-workers predict that the most stable form of C,H, + is the 2-propyl cation 98.
The second most stable structure they found to be the corner-protonated cyclo-
A
98
propane, 97. The calculations suggest that 97 lies at an energy minimum and
thus is an intermediate, not merely a transition state, but that 96 is probably
not an intermediate. Figure 6.18 shows some of the structures and their calcu-
lated relative energies.121
A number of other reactions have been postulated to involve protonated
cyclopropanes as intermediates.lZ2 For example, nmr studies of the sec-butyl
cation in superacid show that from - 100 to - 40°C a process, with an activation
energy of 7 kcal mole-l, occurs that scrambles all the protons. The activation
energy is too low for the scrambling to occur by the mechanism shown in Equa-
IZ1 P. C. Hariharan, L. Radoni, J. A. Pople, P. v. R. Schleyer, J. Amer. Chem. Soc., 96, 599 (1974).
For a recent example, see: C. H. DePuy, A. H. Andrist, and P. C. Fiinfschilling, J. Amer. Chem.
Soc., 96, 948 (1974).