Page 321 - Mechanism and Theory in Organic Chemistry
P. 321
.
I50
100 '
counts sec-' 1 50 .
0.
-
(eV)
Eb
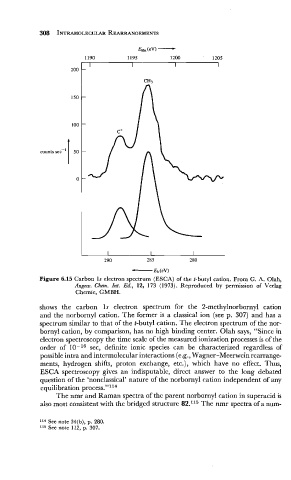
Figure 6.15 Carbon 1s electron spectrum (ESCA) of the t-butyl cation. From G. A. Olah,
Angew. Chem. Znt. Ed., 12, 173 (1973). Reproduced by permission of Verlag
Chemie, GMBH.
shows the carbon 1s electron spectrum for the 2-methylnorbornyl cation
and the norbornyl cation. The former is a classical ion (see p. 307) and has a
spectrum similar to that of the t-butyl cation. The electron spectrum of the nor-
bornyl cation, by comparison, has no high binding center. Olah says, "Since in
electron spectroscopy the time scale of the measured ionization processes is of the
order of 10-l6 sec, definite ionic species can be characterized regardless of
possible intra and intermolecular interactions (e.g., Wagner-Meerwein rearrange-
ments, hydrogen shifts, proton exchange, etc.), which have no effect. Thus,
ESCA spectroscopy gives an indisputable, direct answer to the long debated
question of the 'nonclassical' nature of the norbornyl cation independent of any
equilibration process."l14
The nmr and Raman spectra of the parent norbornyl cation in superacid is
also most consistent with the bridged structure 82.115 The nmr spectra of a num-
"4 See note 34(b), p. 280.
"5 See note 112, p. 307.