Page 323 - Mechanism and Theory in Organic Chemistry
P. 323
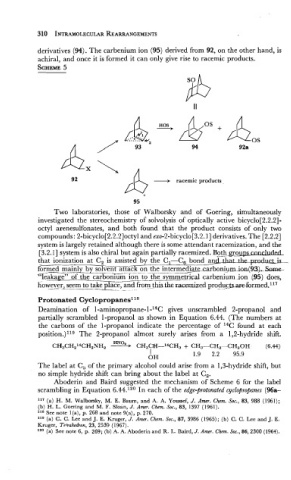
derivatives (94). The carbenium ion (95) derived from 92, on the other hand, is
achiral, and once it is formed it can only give rise to racemic products.
SCHEME
5
Two laboratories, those of Walborsky and of Goering, simultaneously
investigated the stereochemistry of solvolysis of optically active bicyclo[2.2.2]-
octyl arenesulfonates, and both found that the product consists of only two
compounds : 2-bicyclo[2.2.2]octyl and exo-2-bicyclo [3.2.1] derivatives. The [2.2.2]
system is largely retained although there is some attendant racemization, and the
[3.2.1] system is also chiral but again partially racemized. Both .. groups mn&&d
that ionization at C2 is assisted by the Cl-C, bond an- the 1s-
Grmed mainly_bysolv~ttaE&n ?-~ the-bonigmion(=- --
-
the
"1eag~2~of the carbonmm Ion to ___.____ symmetrical carbeni'um ion (95) does,
._.-
to
lib&~v_ec2_seem take place, . - and . frornthkthe racemized prod~c~forrned.~~~
.
.
-
-
Protonated Cyc10propane.s~~~
Deamination of 1 -aminopropane- 1 -14C gives unscrambled 2-propanol and
partially scrambled 1-propanol as shown in Equation 6.44. (The numbers at
the carbons of the 1-propanol indicate the percentage of 14C found at each
position.)llg The 2-propanol almost surely arises from a 1,2-hydride shift.
HNO.
CH3CH2l4CH2NHZ CH3CH--14CH3 + CH3-CH2-CH20H (6.44)
I 1.9 2.2 95.9
OH
The label at C, of the primary alcohol could arise from a 1,3-hydride shift, but
no simple hydride shift can bring about the label at C,.
Aboderin and Baird suggested the mechanism of Scheme 6 for the label
scrambling in Equation 6.44.120 In each of the edge-protonated cyclopropanes (96a-
'I7 (a) H. M. Walborsky, M. E. Baum, and A. A. Youssef, J. Amer. Chem. Sac., 83, 988 (1961);
(b) H. L. Goering and M. F. Sloan, J. Amer. Chem. Sac., 83, 1397 (1961).
"a See note l(a), p. 268 and note 9(a), p. 270.
'Is (a) C. C. Lee and J. E. Kruger, J. Amer. Chem. Sac., 87, 3986 (1965); (b) C. C. Lee and J. E.
Kruger, Tetrahedron, 23, 2539 (1967).
(a) See note 6, p. 269; (b) A. A. Aboderin and R. L. Baird, J. Amer. Chem. Sac., 86,2300 (1964).