Page 318 - Mechanism and Theory in Organic Chemistry
P. 318
Carbonium Ions 305
norbornyl system. He has argued that the kexo/kend0 rate ratio in solvolyses of
norbornyl derivatives is large, not because kexo is particularly great but because
kendo is particularly small. In his view a 2-endo substituent experiences steric
3
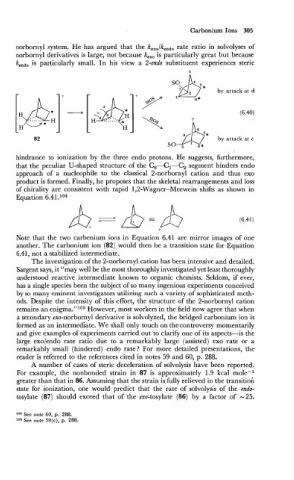
by attack at d
(6.40)
by attack at c
hindrance to ionization by the three endo protons. He suggests, furthermore,
that the peculiar U-shaped structure of the C,-C,-C, segment hinders endo
approach of a nucleophile to the classical 2-norbornyl cation and thus exo
product is formed. Finally, he proposes that the skeletal rearrangements and loss
of chirality are consistent with rapid 1,2-Wagner-Meewein shifts as shown in
Equation 6.41 .Io4
Note that the two carbenium ions in Equation 6.41 are mirror images of one
another. The carbonium ion (82) would then be a transition state for Equation
6.41, not a stabilized intermediate.
The investigation of the 2-norbornyl cation has been intensive and detailed.
Sargent says, it "may well be the most thoroughly investigated yet least thoroughly
understood reactive intermediate known to organic chemists. Seldom, if ever,
has a single species been the subject of so many ingenious experiments conceived
by so many eminent investigators utilizing such a variety of sophisticated meth-
ods. Despite the intensity of this effort, the structure of the 2-norbornyl cation
remains an enigma."lo5 However, most workers in the field now agree that when
a secondary exo-norbornyl derivative is solvolyzed, the bridged carbonium ion is
formed as an intermediate. We shall only touch on the controversy momentarily
and give examples of experiments carried out to clarify one of its aspects-is the
large exolendo rate ratio due to a remarkably large (assisted) exo rate or a
remarkably small (hindered) endo rate? For more detailed presentations, the
reader is referred to the references cited in notes 59.and 60, p. 288.
A number of cases of steric deceleration of solvolysis have been reported.
For example, the nonbonded strain in 87 is approximately 1.9 kcal mole-'
greater than that in 86. Assuming that the strain is fully relieved in the transitioh
state for ionization, one would predict that the rate of solvolysis of the endo-
tosylate (87) should exceed that of the exo-tosylate (86) by a factor of -25.
lo4 See note 60, p. 288.
lo6 See note 59(c), p. 288.