Page 314 - Mechanism and Theory in Organic Chemistry
P. 314
Carbonium Ions 301
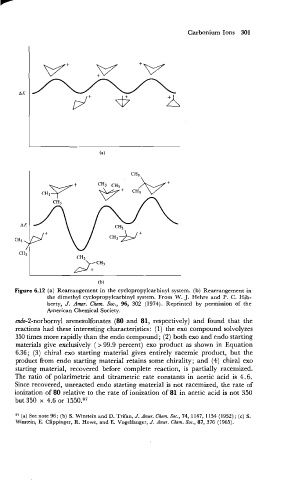
Figure 6.12 (a) Rearrangement in the cyclopropylcarbinyl system. (b) Rearrangement in
the dimethyl cyclopropylcarbinyl system. From W. J. Hehre and P. C. Hib-
berty, J. Amer. Chm. Soc., 96, 302 (1974). Reprinted by permission of the
American Chemical Society.
endo-2-norbornyl arenesulfonates (80 and 81, respectively) and found that the
reactions had these interesting characteristics: (1) the exo compound solvolyzes
350 times more rapidly than the endo compound; (2) both exo and endo starting
materials give exclusively ( > 99.9 percent) exo product as shown in Equation
6.36; (3) chiral exo starting material gives entirely racemic product, but the
product from endo starting material retains some chirality; and (4) chiral exo
starting material, recovered before complete reaction, is partially racemized.
The ratio of polarimetric and titrametric rate constants in acetic acid is 4.6.
Since recovered, unreacted endo starting material is not racemized, the rate of
ionization of 80 relative to the rate of ionization of 81 in acetic acid is not 350
but 350 x 4.6 or 1550.97
O7 (a) See note 96; (b) S. Winstein and D. Trifan, J. Amer. Chem. Soc., 74, 1147, 1154 (1952); (c) S.
Winstein, E. Clippinger, R. Howe, and E. Vogelfanger, J. Amer. Chem. SOC., 87, 376 (1965).