Page 312 - Mechanism and Theory in Organic Chemistry
P. 312
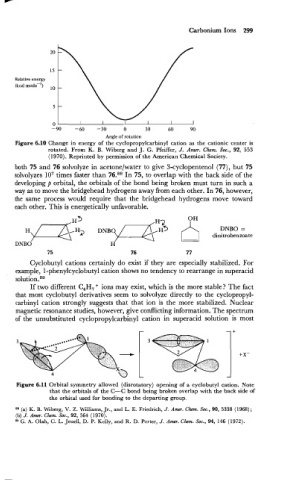
Carbonium Ions 299
Relative energy
(kcal mode-')
Angle of rotation
Figure 6.10 Change in energy of the cyclopropylcarbinyl cation as the cationic center is
rotated. From K. B. Wiberg and J. G. Pfeiffer, J. Amer. Chem. Soc., 92, 553
(1 970). Reprinted by permission of the American Chemical Society.
both 75 and 76 solvolyze in acetonelwater to give 3-cyclopentenol (77), but 75
solvolyzes lo7 times faster than 76.88 In 75, to overlap with the back side of the
developing p orbital, the orbitals of the bond being broken must turn in such a
way as to move the bridgehead hydrogens away from each other. In 76, however,
the same process would require that the bridgehead hydrogens move toward
each other. This is energetically unfavorable.
Cyclobutyl cations certainly do exist if they are especially stabilized. For
example, 1-phenylcyclobutyl cation shows no tendency to rearrange in superacid
solution.89
If two different C,H7+ ions may exist, which is the more stable? The fact
that most cyclobutyl derivatives seem to solvolyze directly to the cyclopropyl-
carbinyl cation strongly suggests that that ion is the more stabilized. Nuclear
magnetic resonance studies, however, give conflicting information. The spectrum
of the unsubstituted cyclopropylcarbinyl cation in superacid solution is most
Figure 6.11 Orbital symmetry allowed (disrotatory) opening of a cyclobutyl cation. Note
that the orbitals of the C-C bond being broken overlap with the back side of
the orbital used for bonding to the departing group.
O8 (a) K. B. Wiberg, V. Z. Williams, Jr., and L. E. Friedrich, J. Amer. Chem. Soc., 90, 5338 (1968);
(b) J. Amer. Chem. Soc., 92, 564 (1970).
G. A. Olah, C. L. Jeuell, D. P. Kelly, and R. D. Porter, J. Amer. Chem. Soc., 94, 146 (1972).