Page 310 - Mechanism and Theory in Organic Chemistry
P. 310
Carbonium Ions 297
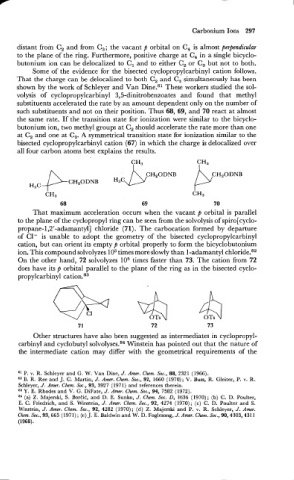
distant from C, and from C,; the vacant p orbital on C4 is almost perpendicular
to the plane of the ring. Furthermore, positive charge at C4 in a single bicyclo-
butonium ion can be delocalized to C, and to either C, or C, but not to both.
Some of the evidence for the bisected cyclopropylcarbinyl cation follows.
That the charge can be delocalized to both C, and C, simultaneously has been
shown by the work of Schleyer and Van Dine.s1 These workers studied the sol-
volysis of cyclopropylcarbinyl 3,5-dinitrobenzoates and found that methyl
substituents accelerated the rate by an amount dependent only on the number of
such substituents and not on their position. Thus 68, 69, and 70 react at almost
the same rate. If the transition state for ionization were similar to the bicyclo-
butonium ion, two methyl groups at C, should accelerate the rate more than one
at C, and one at C,. A symmetrical transition state for ionization similar to the
bisected cyclopropylcarbinyl cation (67) in which the charge is delocalized over
all four carbon atoms best explains the results.
7H3 CH,
I
P H3C \D/ CHzODNB
CH20DNB
CH,ODNB
H3C CH3 P
CH3
68 69 70
That maximum acceleration occurs when the vacant p orbital is parallel
to the plane of the cyclopropyl ring can be seen from the solvolysis of spiroCcyclo-
propane-1,2'-adamantyl] chloride (71). The carbocation formed by departure
of C1- is unable to adopt the geometry of the bisected cyclopropylcarbinyl
cation, but can orient its empty p orbital properly to form the bicyclobutonium
ion. This compound solvolyzes lo3 times more slowly than 1-adamantyl chloride.82
On the other hand, 72 solvolyzes lo5 times faster than 73. The cation from 72
does have its p orbital parallel to the plane of the ring as in the bisected cyclo-
propylcarbinyl cation.83
Other structures have also been suggested as intermediates in cyclopropyl-
carbinyl and cyclobutyl s~lvolyses.~~ Winstein has pointed out that the nature of
the intermediate cation may differ with the geometrical requirements of the
P. v. R. Schleyer and G. W. Van Dine, J. Amer. Chem. Soc., 88, 2321 (1966).
B. R. Ree and J. C. Martin, J. Amer. Chem. Soc., 92, 1660 (1970); V. Buss, R. Gleiter, P. v. R.
Schleyer, J. Amer. Chem. Soc., 93, 3927 (1971) and references therein.
Y. E. Rhodes and V. G. DiFate, J. Amer. Chem. Soc., 94, 7582 (1972).
O4 (a) Z. Majerski, S. BorEiC, and D. E. Sunko, J. Chem. Soc. D, 1636 (1970); (b) C. D. Poulter,
E. C. Friedrich, and S. Winstein, J. Amer. Chem. Soc., 92, 4274 (1970); (c) C. D. Poulter and S.
Winstein, J. Amer. Chem. Soc., 92, 4282 (1970); (d) Z. Majerski and P. v. R. Schleyer, J. Amer.
Chem. Soc., 93, 665 (1971); (e) J. E. Baldwin and W. D. Foglesong, J. Amer. Chem. Soc., 90,4303, 431 1
(1968).