Page 309 - Mechanism and Theory in Organic Chemistry
P. 309
Note that if the bicyclobutonium ion were formed directly upori ionization
of an allylcarbinyl derivative, it would be a case of homoallylic participation in
an acyclic system. In fact, the bicyclobutonium ion is similar to the carbonium ion
proposed by Winstein for homoallylic participation in the 7-norbornenyl system-
cf. Figures 6.7 and 6.8 and Structures 65 and 66. The difference between them is
that 66 is more symmetrical.
Since Roberts' work, _a great deal of evidence, - - - -. both -- experham1 and
theoretical, has accumulated that inxates - tha-thdicyclohutonium ion is not
--
the first-formed ion upon solvolysis of unstrained cyclopropylcarhinyl systems.
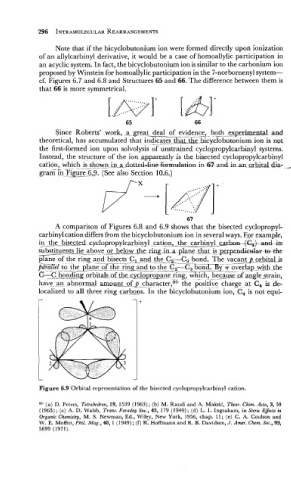
Instead, the structure of the ion apparently is the bisected cyclopropylcarbinyl
. _ . is shown --- adW&~f&tien
cation, which _ in in 67 andinanarlital dia-. -a
gram GI Figure 6.9. (See also Section 10.6.)
-.
67
A comparison of' Figures 6.8 and 6.9 shows that the bisected cyclopropyl-
carbinyl cation differs from the bicyclobutonium ion in several ways. F-or example,
i_n the bisected cyclopropylcarbinyl catioxthe carbinyl ~&~~+@&)-dits
substituents lie above or below the ring inaplanethat is gxqxmbah* +he
plane of the ring and bisects C,-and the C,-C3 bond. The vacant-b orbi~alis
-- --
to
the
pG7iZZel to the plane of the ring and -- --- C,-C3 bond. By G &Gfap-with.the
-
-
-
--
-
-
C-L-cC bGnzng .--- orbitals of the cyclopr~ane ring, which, - because of anglestrain,
have-.an abnorm-a! amQunt ofp chara~ter,~' the positive charge at C4 is de-
localized to all three ring carb-oi. 1n the bicyclobutonium ion, C4 is not equi-
Figure 6.9 Orbital representation of the bisected cyclopropylcarbinyl cation.
80 (a) D. Peters, Tetrahedron, 19, 1539 (1963); (b) M. Randi and A. Maksid, Theor. Chim. Acta, 3, 59
(1965); (c) A. D. Walsh, Trans. Faraday Soc., 45, 179 (1949); (d) L. I. Ingraham, in Steric Effects in
Organic Chemistry, M. S. Newman, Ed., Wiky, New York, 1956, chap. 11 ; (e) C. A. Coulson and
W. E. Moffitt, Phil. Mag., 40, 1 (1949); (f) R. Hoffmann and R. B. Davidson, J. Amer. Chem. Soc., 93,
5699 (1971).