Page 304 - Mechanism and Theory in Organic Chemistry
P. 304
Carbonium Ions 291
step, form either the rearranged carbenium ion 50 or the same carbonium ion
formed in the solvolysis of 47. Thus both compounds give the same product.67
A much more spectacular driving force was found in the acetolysis of anti-7-
norbornenyl tos~late (51). This compound solvolyzes 1Ol1 times faster than the
saturated analog and gives as the sole product the anti-acetate, 52.68 Winstein
attributed the enormously accelerated rate to powerful anchimeric assistance of
both p orbitals of the 2,3-double bond.
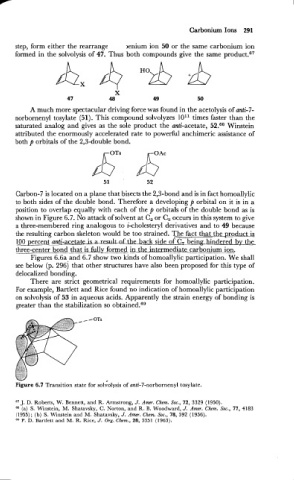
Carbon-7 is located on a plane that bisects the 2,3-bond and is in fact homoallylic
to both sides of the double bond. Therefore a developing p orbital on it is in a
position to overlap equally with each of the p orbitals of the double bond as is
shown in Figure 6.7. No attack of solvent at C, or C, occurs in this system to give
a three-membered ring analogous to i-cholesteryl derivatives and to 49 because
the resulting carbon skeleton would be too strained. The fact that the product is
100 percent anti-acetateisad& back side of C, being. hindered by the
three-center bond that is fullyfrmed in the intermediakcarbonium ion.
Figures 6.6a and 6.7 show two kinds of homoallylic participation. We shall
see below (p. 296) that other structures have also been proposed for this type of
delocalized bonding.
There are strict geometrical requirements for homoallylic participation.
For example, Bartlett and Rice found no indication of homoallylic participation
on solvolysis of 53 in aqueous acids. Apparently the strain energy of bonding is
greater than the stabilization so obtained.69
87 J. D. Roberts, W. Bennett, and R. Armstrong, J. Amer. Chem. Soc., 72, 3329 (1950).
88 (a) S. Winstein, M. Shatavsky, C. Norton, and R. B. Woodward, J. Amer. Chem. Soc., 77, 4183
(1955); (b) S. Winstein and M. Shatavsky, J. Amer. Chem. Soc., 78, 592 (1956).
68 P. D. Bartlett and M. R. Rice, J. Org. Chem., 28, 3351 (1963).