Page 302 - Mechanism and Theory in Organic Chemistry
P. 302
Carbonium Ions 289
a 3 AcO
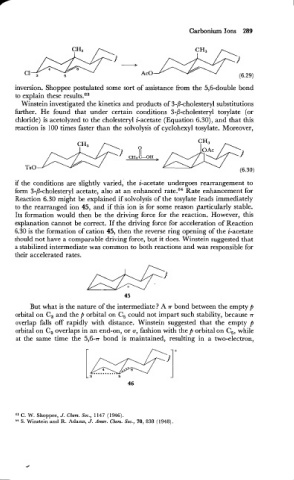
inversion. Shoppee postulated some sort of assistance from the 5,6-double bond
to explain these resu1tseG3
Winstein investigated the kinetics and products of 3-P-cholesteryl substitutions
further. He found that under certain conditions 3-P-cholesteryl tosylate (or
chloride) is acetolyzed to the cholesteryl i-acetate (Equation 6.30), and that this
reaction is 100 times faster than the solvolysis of cyclohexyl tosylate. Moreover,
if the conditions are slightly varied,' the i-acetate undergoes rearrangement to
form 3-/I-cholesteryl acetate, also at an enhanced rate." Rate enhancement for
Reaction 6.30 might be explained if solvolysis of the tosylate leads immediately
to the rearranged ion 45, and if this ion is for some reason particularly stable.
Its formation would then be the driving force for the reaction. However, this
explanation cannot be correct. If the driving force for acceleration of Reaction
6.30 is the formation of cation 45, then the reverse ring opening of the i-acetate
should not have a comparable driving force, but it does. Winstein suggested that
a stabilized intermediate was common to both reactions and was responsible for
their accelerated rates.
But what is the nature of the intermediate? A .rr bond between the empty p
orbital on C, and the P orbital on C, could not impart such stability, because .rr
overlap falls off rapidly with distance. Winstein suggested that the empty p
orbital on C, overlaps in an end-on, or a, fashion with the p orbital on C,, while
at the same time the 5,6-.rr bond is maintained, resulting in a two-electron,
83 C. W. Shoppee, J. Chem. SOL, 1147 (1946).
u4 S. Winstein and R. Adams, J. Amer. Chem. SOL, 70, 838 (1948).