Page 298 - Mechanism and Theory in Organic Chemistry
P. 298
1,2-Shifts in Carbenium Ions 285
mously from values in another reaction or even in the same reaction under other
conditions.
Both "intermolecular" and intramolecular migratory aptitudes have been
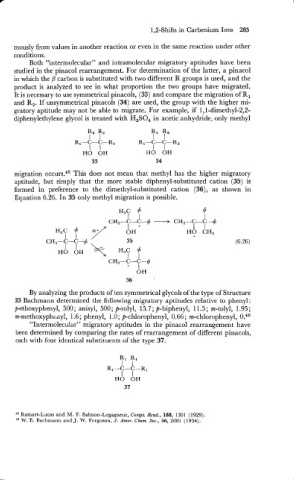
studied in the pinacol rearrangement. For determination of the latter, a pinacol
in which the P carbon is substituted with two different R groups is used, and the
product is analyzed to see in what proportion the two groups have migrated.
It is necessary to use symmetrical pinacols, (33) and compare the migration of R,
and R,. If unsymmetrical pinacols (34) are used, the group with the higher mi-
gratory aptitude may not be able to migrate. For example, if 1,l-dimethyl-2,2-
diphenylethylene glycol is treated with H2S04 in acetic anhydride, only methyl
migration occurs.45 This does not mean that methyl has the higher migratory
aptitude, but simply that the more ' stable diphenyl-substituted cation (35) is
formed in preference to the dimethyl-substituted cation (36), as shown in
Equation 6.26. In 35 only methyl migration is possible.
By analyzing the products of ten symmetrical glycols of the type of Structure
33 Bachmann determined the following migratory aptitudes relative to phenyl :
p-ethoxyphenyl, 500 ; anisyl, 500; p-tolyl, 15.7 ; p-biphenyl, 1 1.5 ; m-tolyl, 1.95 ;
m-methoxyph~ily!, 1.6 ; phenyl, 1 .O; p-chlorophenyl, 0.66 ; m-chlorophenyl, 0.46
"Intermolecular" migratory aptitudes in the pinacol rearrangement have
been determined by comparing the rates of rearrangement of different pinacols,
each with four identical substituents of the type 37.
45 Ramart-Lucas and M. F. Salmon-Legagneur, Compt. Rend., 188, 1301 (1929).
46 W. E. Bachmann and J. W. Ferguson, J. Amer. Chem. Soc., 56, 2081 (1934).