Page 296 - Mechanism and Theory in Organic Chemistry
P. 296
1,f-Shifts in Carbenium Ions 283
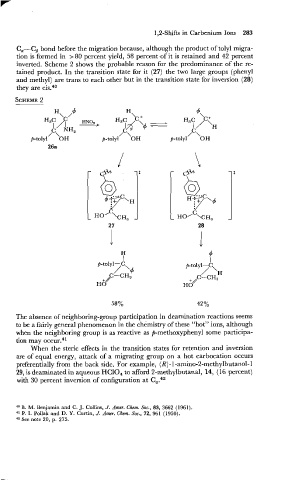
C,-CB bond before the migration because, although the product of tolyl migra-
tion is formed in > 80 percent yield, 58 percent,of it is retained and 42 percent
inverted. Scheme 2 shows the probable reason for the predominance of the re-
tained product. In the transition state for it (27) the two large groups (phenyl
and methyl) are trans to each other but in the transition state for inversion (28)
they are cis.40
p-tolyl- C
/ "4
+/c-CH3
HO
The absence of neighboring-group participation in deamination reactions seems
to be a fairly general phenomenon in the chemistry of these "hot" ions, although
when the neighboring group is as reactive as p-methoxyphenyl some participa-
tion may occur.'l
When the steric effects in the transition states for retention and inversion
are of equal energy, attack of a migrating group on a hot carbocation occurs
preferentially from the back side. For example, (R)- 1 -amino-2-methylbutanol- 1
29, is deaminated in aqueous HClO, to afford 2-methylbutanal, 14, (16 percent)
with 30 percent inversion of configuration at C,.42
40 B. M. Benjamin and C. J. Collins, J. Amer. Chem. Soc., 83, 3662 (1961).
" P. I. Pollak and D. Y. Curtin, J. Amr. Chem. Soc., 72, 961 (1950).
'2 See note 20, p. 275.