Page 292 - Mechanism and Theory in Organic Chemistry
P. 292
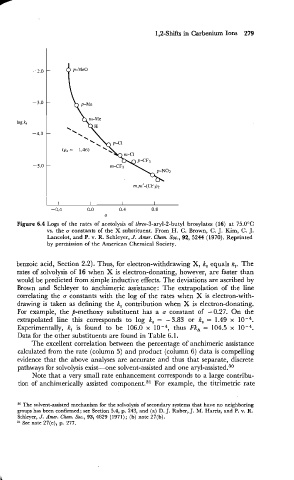
1,2-Shifts in Carbenium Ions 279
Figure 6.4 Logs of the rates of acetolysis of threo-3-aryl-2-butyl brosylates (16) at 75.0°C
vs. the o constants of the X substituent. FromH. C. Brown, C. J. Kim, C. J.
Lancelot, and P. v. R. Schleyer, J. Amer. Chm. SOG., 92, 5244 (1970). Reprinted
by permission of the American Chemical Society.
benzoic acid, Section 2.2). Thus, for electron-withdrawing X, k, equals kt. The
rates of solvolysis of 16 when X is electron-donating, however, are faster than
would be predicted from simple inductive effects. The deviations are ascribed by
Brown and Schleyer to anchimeric assistance: The extrapolation of the line
correlating the a constants with the log of the rates when X is electron-with-
drawing is taken as defining the k, contribution when X is electron-donating.
For example, the p-methoxy substituent has a a constant of -0.27. On the
extrapolated line this corresponds to log k, = -3.83 or k, = 1.49 x
Experimentally, kt is found to be 106.0 x thus FkA = 104.5 x
Data for the other substituents are found in Table 6.1.
The excellent correlation between the percentage of anchimeric assistance
calculated from the rate (column 5) and product (column 6) data is compelling
evidence that the above analyses are accurate and thus that separate, discrete
pathways for solvolysis exist-one solvent-assisted and one aryl-assisted.30
Note that a very small rate enhancement corresponds to a large contribu-
tion of anchimerically assisted c~mponent.~~ For example, the titrimetric rate
30 The solvent-assisted mechanism for the solvolysis of secondary systems that have no neighboring
groups has been confirmed; see Section 5.4, p. 243, and (a) D. J. Raber, J. M. Harris, and P. v. R.
Schleyer, J. Amer. Chem. Soc., 93, 4829 (1971); (b) note 27(b).
31 See note 27(c), p. 277.