Page 290 - Mechanism and Theory in Organic Chemistry
P. 290
1,2-Shifts in Carbenium Ions 277
by watching the rate of loss of optical activity. He found that the polarimetric
rate was five times the titrametric rate and concluded that the intermediate
phenonium ion is formed rapidly but reverts back to starting materials four times
more often than it goes on to products. That both solvolysis and racemization
occur through a common intermediate seeemed most probable because of the
similar sensitivity of the rates of both reactions to solvent polarity.24
The concept of an intermediate phenonium ion was, at first, controversial,
and its chief detractor was H. C. Brown.25 Although 3-phenyl-2-butyl tosylate
showed the stereochemical behavior expected if an intermediate phenonium ion
were formed, it did not, in his opinion, show the rate acceleration that should
attend anchimeric assistance to ionization of the to~ylate.~~ Brown said that the
stereochemical results could be accounted for by invoking rapidly equilibrating
open carbocations (15). According to his explanation, ionization of the tosylate
H3C CH3 H3C CH3
I I I I
H-C-C-H H-C-C-H
occurs, for steric reasons, only when the phenyl and the tosylate are trans to each
other. The phenyl then migrates rapidly back and forth, blocking solvent attack
from the back side by the "windshield wiper effect." Rotation about the C,-C,
bond does not occur because (1) the rapid phenyl transfer hinders it and (2) the
large phenyl group must remain trans to the large, departing, tosylate group.
Solvent attack from the back side, relative to the tosylate, is blocked by the
phenyl group. Eventually, solvent attacks the a or #3 carbon from the front side,
giving the stereochemical results obtained by Cram and by Winstein.
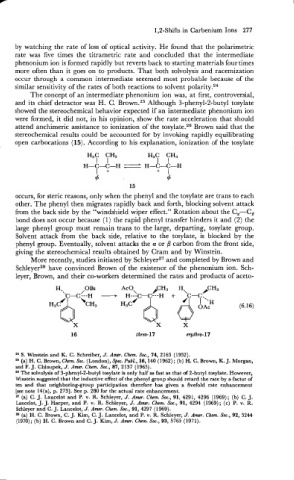
More recently, studies initiated by SchleyerZ7 and completed by Brown and
SchleyerZ8 have convinced Brown of the existence of the phenonium ion. Sch-
leyer, Brown, and their co-workers determined the rates and products of aceto-
. /
H., OBs
C-C---H +
(6.16)
X X X
S. Winstein and K. C. Schreiber, J. Amer. Chem. Soc., 74, 2165 (1952).
'' (a) H. C. Brown, Chem. Soc. (London), Spec. Publ., 16, 140 (1962); (b) H. C. Brown, K. J. Morgan,
and F. J. Chloupek, J. Am. Chem. Soc., 87, 2137 (1965).
The solvolysis of 3-phenyl-2-butyl tosylate is only half as fast as that of 2-butyl tosylate. However,
Winstein suggested that the inductive effect of the phenyl group should retard the rate by a factor of
ten and that neighboring-group participation therefore has given a fivefold rate enhancement
[see note 14(a), p. 2731. See p. 280 for the actual rate enhancement.
(a) C. J. Lancelot and P. v. R. Schleyer, J. Am. Chem. SOC., 91, 4291, 4296 (1969); (b) C. J.
Lancelot, J. J. Harper, and P. v. R. Schleyer, J. Amer. Chem. Soc., 91, 4294 (1969); (c) P. v. R.
Schleyer and C. J. Lancelot, J. Amer. Chem. Soc., 91, 4297 (1969).
an (a) H. C. Brown, C. J. Kim, C. J. Lancelot, and P. v. R. Schleyer, J. Am. Chem. Soc., 92, 5244
(1970); (b) H. C. Brown and C. J. Kim, J. Amer. Chem. Soc., 93, 5765 (1971).