Page 289 - Mechanism and Theory in Organic Chemistry
P. 289
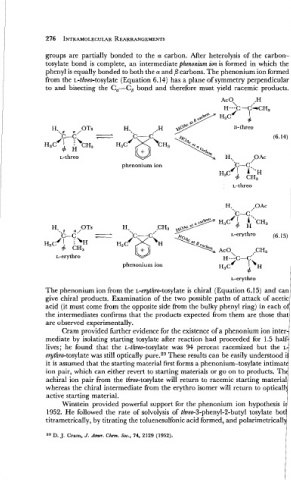
groups are partially bonded to the a carbon. After heterolysis of the carbon-
tosylate bond is complete, an intermediate phenonium ion is formed in which the
phenyl is equally bonded to both the a and ,B carbons. The phenonium ion formed
from the L-threo-tosylate (Equation 6.14) has a plane of symmetry perpendicular
to and bisecting the C,-C, bond and therefore must yield racemic products.
Ac 0, .,H
H--)c-c'-CH,
H3C 4 I
4
OTs H. D-threo
C-C (6.14)
L-threo H,, ,OAc
phenonium ion 'c-c:
'1 A ''H
H3C 4 CH,
OTs
H'.,D 01,
c-c
H3C'I :'H
4 CH3
phenonium ion
The phenonium ion from the L-e ythro-tosylate is chiral (Equation 6.15) and can
give chiral products. Examination of the two possible paths of attack of acetic
acid (it must come from the opposite side from the bulky phenyl ring) in eachof
the intermediates confirms that the products expected from them are those that
are observed experimentally.
Cram provided further evidence for the existence of a phenonium ion inter-
mediate by isolating starting tosylate after reaction had proceeded for 1.5 half-
lives; he found that the L-threo-tosylate was 94 percent racemized but the L-
erythro-tosylate was still optically pure.23 These results can be easily understood ii
it is assumed that the starting material first forms a phenonium-tosylate intimate
ion pair, which can either revert to starting materials or go on to products. The
achiral ion pair from the threo-tosylate will return to racemic starting material
whereas the chiral intermediate from the erythro isomer will return to opticall)
active starting material.
Winstein provided powerful support for the phenonium ion hypothesis ir
1952. He followed the rate of solvolysis of threo-3-phenyl-2-butyl tosylate botl
titrametrically, by titrating the toluenesulfonic acid formed, and polarimetrically
a3 D. J. Cram, J. Am. Chem. Soc., 74, 2129 (1952).