Page 286 - Mechanism and Theory in Organic Chemistry
P. 286
1,2-Shifks in Carbenium Ions 273
hydrogen have no unshared pairs: The pair of electrons the migrating group
takes with it from the /3 to the a carbon is partially available to the a carbon at the
transition state for the migration, as illustrated in Structure 7 and in Figure 6.2.
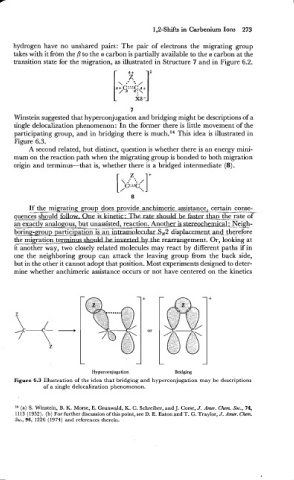
7
Winstein suggested that hyperconjugation and bridging might be descriptions of a
single delocalization phenomenon : In the former there is little movement of the
participating group, and in bridging there is much.14 This idea is illustrated in
Figure 6.3.
A second related, but distinct, question is whether there is an energy mini-
mum on the reaction path when the migrating group is bonded to both migration
origin and terminus-that is, whether there is a bridged intermediate (8).
-
If the migratina group does provide anchimeric assistance, certain conse-
---
uence~should fo bcmdku%&Oneiskinetic:ate should he faster than the rate of
!n exactly analogous, but unassisted, reaction. Another i-hemical : N&-
boring-.group participation is an iut-.S,2 displacement and therefore
the migration t e r m i n u s hy thy rearrangement. Or, looking at
it another way, two closely related molecules may react by different paths if in
one the neighboring group can attack the leaving group from the back side,
but in the other it cannot adopt that position. Most experiments designed to deter-
mine whether anchimeric assistance occurs or not have centered on the kinetics
+
Hyperconjugation Bridging
Figure 6.3 Illustration of the idea that bridging and hyperconjugation may be descriptions
of a single delocalization phenomenon.
l4 (a) S. Winstein, B. K. Morse, E. Grunwald, K. C. Schreiber, and J. Corse, J. Amer. Chem. SOC., 74,
11 13 (1952). (b) For further discussion of this point, see D. E. Eaton acd T. G. Traylor, J. Amer. Chem.
Sac., 96, 1226 (1974) and references therein.