Page 287 - Mechanism and Theory in Organic Chemistry
P. 287
and/or stereochemistry of the reaction under consideration. Rate acceleration is
often difficult to ascertain because of problems in predicting the rate of the non-
assisted reaction. Inversion of configuration is, of course, experimentally ob-
servable only in chiral systems, but in systems that are achiral the stereochemistry
of the reaction can often be determined by isotope labeling.
Experiments indicate that in open-chain and unstrained cyclic compounds,
hydride and alkyl groups usually do not provide anchimeric assistance if the
leaving group is on a secondary or tertiary carbon.15 (For a discussion of partici-
pation in strained cyclic systems, see Section 6.2.) Early evidence against neigh-
boring-group participation by alkyl groups came from oxygen-exchange studies
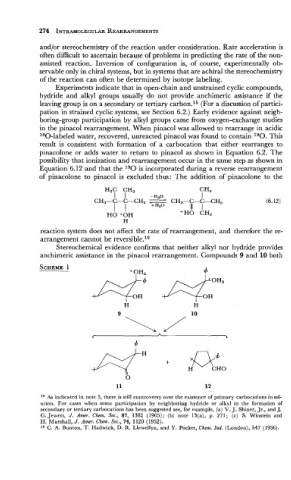
in the pinacol rearrangement. When pinacol was allowed to rearrange in acidic
180-labeled water, recovered, unreacted pinacol was found to contain 180. This
result is consistent with formation of a carbocation that either rearranges to
pinacolone or adds water to return to pinacol as shown in Equation 6.2. The
possibility that ionization and rearrangement occur in the same step as shown in
Equation 6.12 and that the 180 is incorporated during a reverse rearrangement
of pinacolone to pinacol is excluded thus: The addition of pinacolone to the
reaction system does not affect the rate of rearrangement, and therefore the re-
arrangement cannot be reversible.16
Stereochemical evidence confirms that neither alkyl nor hydride provides
anchimeric assistance in the pinacol rearrangement. Compounds 9 and 10 both
l6 AS indicated in note 5, there is still controversy over the existence of primary carbocations in sol-
ution. For cases when some participation by neighboring hydride or alkyl in the formation of
secondary or tertiary carbocations has been suggested see, for example, (a) V. J. Shiner, Jr., and J.
G. Jewett, J. Amer. Chem. Soc., 87, 1382 (1965); (b) note 13(a), p. 271 ; (c) S. Winstein and
H. Marshall, J. Amer. Chem. Soc., 74, 1120 (1952).
l6 C. A. Bunton, T. Hadwick, D. R. Llewellyn, and Y. Pocker, Chem. Znd. (London), 547 (1956).