Page 284 - Mechanism and Theory in Organic Chemistry
P. 284
1,2-Shifts in Carbenium Ions 271
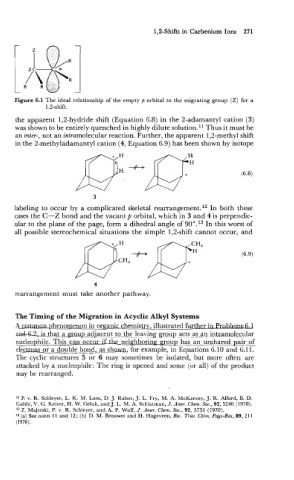
Figure 6.1 The ideal relationship of the empty p orbital to the migrating group (Z) for a
1,2-shift.
the apparent 1,2-hydride shift (Equation 6.8) in the 2-adamantyl cation (3)
was shown to be entirely quenched in highly dilute solution.ll Thus it must be
an inter-, not an intramolecular reaction. Further, the apparent 1,2-methyl shift
in the 2-methyladamantyl cation (4, Equation 6.9) has been shown by isotope
labeling to occur by a complicated skeletal rearrangement.12 In both these
cases the C-Z bond and the vacant p orbital, which in 3 and 4 is perpendic-
ular to the plane of the page, form a dihedral angle of 90°.13 In this worst of
all possible stereochemical situations the simple 1,2-shift cannot occur, and
rearrangement must take another pathway.
The Timing of the Migration in Acyclic Alkyl Systems
Acommon~henomenon in organic chemistry, illustrated further in PrablemsG.1
amkU&-that a sroup adiacent to t& leaving group acts as-a~ intramolecular
nucleophile. This can occur if the neighboring Froup has an unshared pair of
elsnns or a double ----- bond - as - shm, - for example, in Equations 6.10 and 6.1 1.
The cyclic structures 5 or 6 may sometimes be isolated, but more often are
attacked by a nucleophile: The ring is opened and some (or all) of the product
may be rearranged.
l1 P. v. R. Schleyer, L. K. M. Lam, D. J. Raber, J. L. Fry, M. A. McKervey, J. R. Alford, B. D.
Cuddy, V. G. Keizer, H. W. Geluk, and J. L. M. A. Schlatman, J. Amer. Chem. Soc., 92, 5246 (1970).
l2 2. Majerski, P. v. R. Schleyer, and A. P. Wolf, J. Amer. Chem. Soc., 92, 5731 (1970).
'3 (a) See notes 11 and 12; (b) D. M. Brouwer and H. Hogeveen, Rec. Trav. Chitn. Pays-Bas, 89, 21 1
(1970).