Page 285 - Mechanism and Theory in Organic Chemistry
P. 285
Bec-
- mentratian~ af the neighborin~isJr.ery. high iit is
always inthckmmediate vicinity of fiereaction site) and because o_fthe.relati,vely
small dzree .~ reorganization required to reach-&-en-sate (and,
of
-
of this type are ofte~ faster tha*xpter-
therefore, small~entropy change), . . - .--- reactions .
molecular or unimolecular substitutions. .- The nucleophilic assistance of a neigh-
-.-.
boring group to departiofaTeaGing group is called neighboripg group participation
or anchimeric assistance.
Analogy with intramolecular nucleophilic substitution reactions raises two
fundamental problems in the study of rearrangements. The first is whether, mhg
an
xarbon or hwa~esin electrpn-deficient structure, - -. it does SQ dy
.
after the cationic center has fully formed in a previous step, or whetha-$-..mi-
. ..- -. -- , . . - ~
grates simultaneously with uc the leavin~rou~ thus--p_rp,~iding
of
anchimerizassistance. Such p%pation is conceivable eventhough carbon and
-__I_C_C_
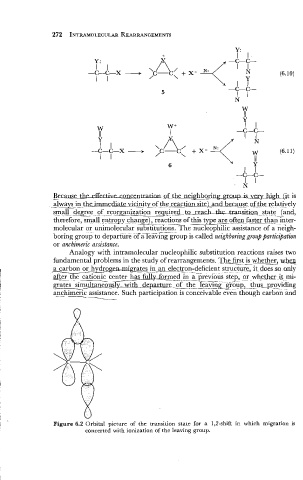
Figure 6.2 Orbital picture of the transition state for a 1,2-shift in which migration is
concerted with ionization of the leaving group.