Page 295 - Mechanism and Theory in Organic Chemistry
P. 295
whether the transition state leading to retention or inversion is more stable. If
they are of equal energy, racemization should result; we have already seen an
example of this in Equation 6.13.
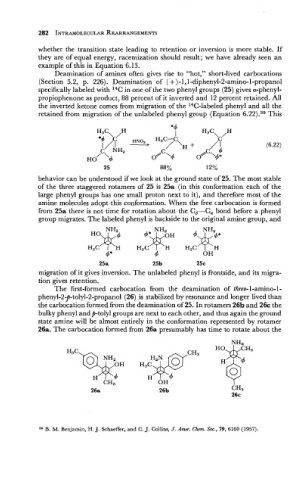
Deamination of amines often gives rise to "hot," short-lived carbocations
(Section 5.2, p. 226). Deamination of ( + )-I, 1-diphenyl-2-amino-l-propanol
specifically labeled with 14C in one of the two phenyl groups (25) gives a-phenyl-
propiophenone as product, 88 percent of it inverted and 12 percent retained. All
the inverted ketone comes from migration of the 14C-labeled phenyl and all the
retained from migration of the unlabeled phenyl group (Equation 6.22).39 This
25 88% 12%
behavior can be understood if we look at the ground state of 25. The most stable
of the three staggered rotamers of 25 is 25a (in this conformation each of the
large phenyl groups has one small proton next to it), and therefore most of the
amine molecules adopt this conformation. When the free carbocation is formed
from 25a there is not time for rotation about the C,-C, bond before a phenyl
group migrates. The labeled phenyl is backside to the original amine group, and
25a 25b 25c
migration of it gives inversion. The unlabeled phenyl is frontside, and its migra-
tion gives retention.
The first-formed carbocation from the deamination of threo-l-amino-l-
phenyl-2-p-tolyl-2-propanol (26) is stabilized by resonance and longer lived than
the carbocation formed from the deamination of 25. In rotamers 26b and 26c the
bulky phenyl andp-tolyl groups are next to each other, and thus again the ground
state amine will be almost entirely in the conformation represented by rotamer
26a. The carbocation formed from 26a presumably has time to rotate about the
38 B. M. Benjamin, H. J. Schaeffer, and C. J. Collins, J. Amer. Chem. Soc., 79, 6160 (1957).