Page 300 - Mechanism and Theory in Organic Chemistry
P. 300
1,2-Shifts in Carbenium Ions 287
Memory Effects53
Heretofore we have been concerned mainly with single rearrangements. Multiple
rearrangements also occur, in which the carbocation formed after the initial
migration rearranges again (and again) before products are formed. Some of
these consecutive rearrangements are remarkable in that presumably identical
carbocations, which arise by rearrangement from different starting materials,
retain a memory of their antecedent and give different second rearrangements.
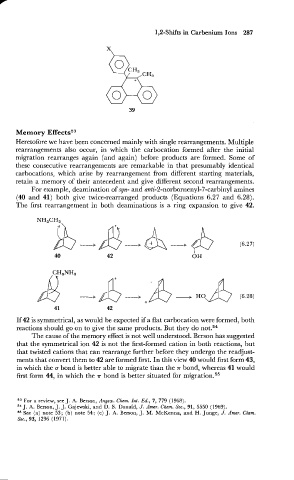
For example, deamination of syn- and anti-2-norbornenyl-7-carbinyl amines
(40 and 41) both give twice-rearranged products (Equations 6.27 and 6.28).
The first rearrangement in both deaminations is a ring expansion to give 42.
If 42 is symmetrical, as would be expected if a flat carbocation were formed, both
reactions should go on to give the same products. But they do not.54
The cause of the memory effect is not well understood. Berson has suggested
that the symmetrical ion 42 is not the first-formed cation in both reactions, but
that twisted cations that can rearrange further before they undergo the readjust-
ments that convert them to 42 are formed first. In this view 40 would first form 43,
in which the o bond is better able to migrate than the .rr bond, whereas 41 would
first form 44, in which the .rr bond is better situated for migration.55
63 For a review, see J. A. Berson, An~gew. Chem. Znt. Ed., 7, 779 (1968).
54 J. A. Berson, J. J. Gajewski, and D. S. Donald, J. Amer. Chem. Soc., 91, 5550 (1969).
65 See (a) note 53; (b) note 54; (c) J. A. Berson, J. M. McKenna, and H. Junge, J. Amer. Chem.
SOC., 93, 1296 (1971).