Page 303 - Mechanism and Theory in Organic Chemistry
P. 303
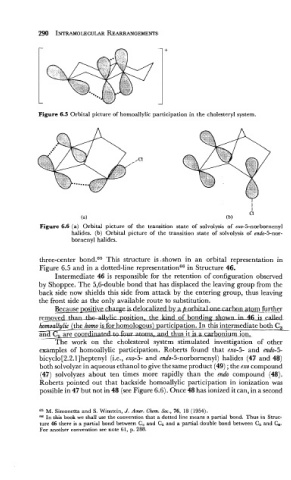
Figure 6.5 Orbital picture of homoallylic participation in the cholesteryl system.
I
I
CI
(a) (b)
Figure 6.6 (a) Orbital picture of the transition state of solvolysis of exo-5-norbornenyl
halides. (b) Orbital picture of the transition state of solvolysis of endo-5-nor-
bornenyl halides.
three-center bond.65 This structure is .shown in an orbital representation in
in
Fi~ure 6.5 and in a dotted-line repre~entation~~ Structure 46.
-
Intermediate 46 is responsible for the retention of configuration observed
by Shoppee. The 5,6-double bond that has displaced the leaving group from the
back side now shields this side from attack by the entering group, thus leaving
the front side as the only available route to substitution.
B-oc.h;l-is dd~cahdhyamcarhm atom further
resovea wnsition. the kd of bonding: shown in 4u_called
homoallyli~the homo is for homologous) participation. In this intermediate both C3
and C, are COO~-; and thus it is ubonium-io-nL
--
The work on the cholesterol system stimulated investigation of other
examples of homoallylic participation. Roberts found that exo-5- and endo-5-
bicyclo[2.2.l]heptenyl (i.e., exo-5- and endo-5-norbornenyl) halides (47 and 48)
both solvolyze in aqueous ethanol to give the same product (49) ; the exo compound
(47) solvolyzes about ten times more rapidly than the endo compound (48).
Roberts pointed out that backside homoallylic participation in ionization was
possible in 47 but not in 48 (see Figure 6.6). Once 48 has ionized it can, in a second
e5 M. Simonetta and S. Winstein, J. Amer. Chem. Soc., 76, 18 (1954).
8e In this book we shall use the convention that a dotted line means a partial bond. Thus in Struc-
ture 46 there is a partial bond between C, and C5 and a partial double bond between C5 and Ce.
For another convention see note 61, p. 288.