Page 308 - Mechanism and Theory in Organic Chemistry
P. 308
Carbonium Ions 295
+
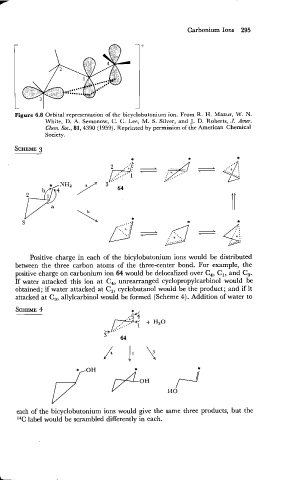
Figure 6.8 Orbital representation of the bicyclobutonium ion. From R. H. Mazur, W. N.
White, D. A. Semonow, C. C. Lee, M. S. Silver, and J. D. Roberts, J. Amer.
Chem. Soc., 81, 4390 (1959). Reprinted by permission of the American Chemical
Society.
Positive charge in each of the bicylobutonium ions would be distributed
between the three carbon atoms of the three-center bond. For example, the
positive charge on carbonium ion 64 would be delocalized over C,, C,, and C,.
If water attacked this ion at C,, unrearranged cyclopropylcarbinol would be
obtained; if water attacked at C,, cyclobutanol would be the product; and if it
attacked at C,, allylcarbinol would be formed (Scheme 4). Addition of water to
each of the bicyclobutonium ions would give the same three products, but the
14C label would be scrambled differently in each.