Page 306 - Mechanism and Theory in Organic Chemistry
P. 306
Carbonium Ions 293
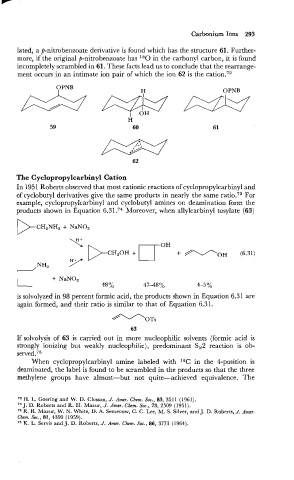
lated, a P-nitrobenzoate derivative is found which has the structure 61. Further-
more, if the original p-nitrobenzoate has 180 in the carbonyl carbon, it is found
incompletely scrambled -in 61. These facts lead us to conclude that the rearrange-
ment occurs in an intimate ion pair of which the ion 62 is the cation.I2
The Cyclopropylcarbinyl Cation
In 195 1 Roberts observed that most cationic reactions of cyclopropylcarbinyl and
of cyclobutyl derivatives give the same products in nearly the same ratio.73 For
example, cyclopropylcarbinyl and cyclobutyl amines on deamination form the
products shown in Equation 6.31.14 Moreover, when allylcarbinyl tosylate (63)
is solvolyzed in 98 percent formic acid, the products shown in Equation 6.3 1 are
again formed, and their ratio is similar to that of Equation 6.31.
If solvolysis of 63 is carried out in more nucleophilic solvents (formic acid is
strongly ionizing but weakly nucleophilic), predominant S,2 reaction is ob-
served.
When cyclopropylcarbinyl amine labeled with 14C in the 4-position is
deaminated, the label is found to be scrambled in the products so that the three
methylene groups have almost-but not quite-achieved equivalence. The
72 H. L. Goering and W. D. Closson, J. Amer. Chem. Soc., 83, 351 1 ( 1961).
73 J. D. Roberts and R. H. Mazur, J. Amer. Chem. Soc., 73, 2509 (1951).
74 R. H. Mazur, W. N. White, D. A. Semenow, C. C. Lee, M. S. Silver, and J. D. Roberts, J. Amer.
Chem. Soc., 81, 4390 (1959).
75 K. L. Servis and J. D. Roberts, J. Amer. Chem. Soc., 86, 3773 (1964).