Page 315 - Mechanism and Theory in Organic Chemistry
P. 315
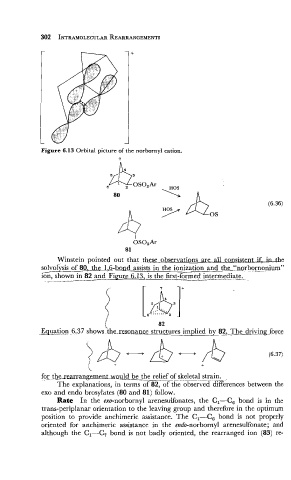
Figure 6.13 Orbital picture of the norbornyl cation.
7
Winstein pointed out that these observations are all consht-$;in_he
solvolisis of 80, the 1,6-bond assists in the ionization and the~''n~~o~nonium"
ion, shown in 82 and Figure 6.13, is the first-formed intermediais.
-
( 82
Equation 6.37 shows the ramance structures implied by 82. The dyiying force
',
--__ _
be
the
-
for - thcrmang.~m~lnt~~uld relief _ of _ skeletal strain. _.
.
The explanations, in terms of 82, of the observed differences between the
exo and endo brosylates (80 and 81) follow.
Rate In the exo-norbornyl arenesulfonates, the Cl-C, bond is in the
trans-periplanar orientation to the leaving group and therefore in the optimum
position to provide anchimeric assistance. The C,-C, bond is not properly
oriented for anchimeric assistance in the endo-norbornyl arenesulfonate; and
although the Cl-C, bond is not badly oriented, the rearranged ion (83) re-