Page 317 - Mechanism and Theory in Organic Chemistry
P. 317
are about 87 percent racemized in strongly nucleophilic solvents. Thus. most of
the product is indeed formed by the reaction path shown in 6 14bLu_t
-
come solvent must also attack C n i u m ion had dm-e-to
. .
r e a r r a n ~ p t s g l 2 vgr-nt ~ of ra~ernIzation.~~~
-
Isomerization of starting material Partial racemization of recovered
exo starting material is consistent with the hypothesis that a,norbornonium ion is
formed immediately on ionization if it is further postulated that an intimate ion
pair is the first ionic species on the reaction path. Internal return from the
achiral norbornonium-arenesulfonate intimate ion pair must give racemized
-
starting: material.lo2
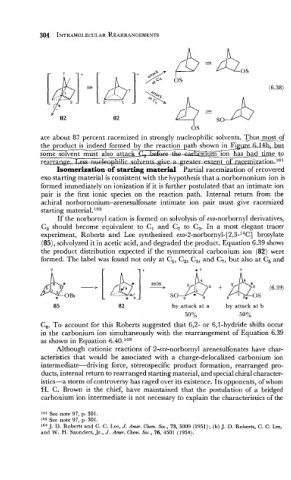
If the norbornyl cation is formed on solvolysis of exo-norbornyl derivatives,
C, should become equivalent to Cl and C, to C,. In a most elegant tracer
experiment, Roberts -and Lee synthesized ex0-2-norborn~l-[2,3-~~~] brosylate
(85), solvolyzed it in acetic acid, and degraded the product. Equation 6.39 shows
the product distribution expected if the symmetrical carbonium ion (82) were
formed. The label was found not only at C,, C,, C,, and C,, but also at C, and
A* [b]+ h5 (6.N)
*
..
.......
-+[
OBs
4'
* b So, * + 6 2* os
by attack at a by attack at b
50 % 50%
C,. To account for this Roberts suggested that 6,2- or 6,l-hydride shifts occur
in the carbonium ion simultaneously with the rearrangement of Equation 6.39
as shown in Equation 6.40.1°3
Although cationic reactions of 2-exo-norbornyl arenesulfonates have char-
acteristics that would be associated with a charge-delocalized carbonium ion
intermediate-driving force, stereospecific product formation, rearranged pro-
ducts, internal return to rearranged starting material, and special chiral character-
istics-a storm of controversy has raged over its existence. Its opponents, of whom
H. C. Brown is the chief, have maintained that the postulation of a bridged
carbonium ion intermediate is not necessary to explain the characteristics of the
lo' See note 97, p. 301.
lo2 See note 97, p. 301.
lo3 J. D. Roberts and C. C. Lee, J. Amer. Chem. Sac., 73, 5009 (1951); (b) J. D. Roberts, C. C. Lee,
and W. H. Saunders, Jr., J. Amer. Chem. Sac., 76, 4501 (1954).