Page 322 - Mechanism and Theory in Organic Chemistry
P. 322
Carbonium Ions 309
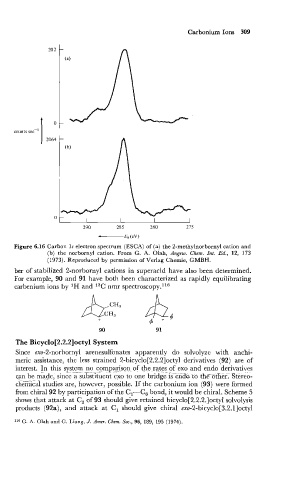
Figure 6.16 Carbon 1s electron spectrum (ESCA) of (a) the 2-methylnorbornyl cation and
(b) the norbornyl cation. From G. A. Olah, Angew. Chem. Int. Ed., 12, 173
(1973). Reproduced by permission of Verlag Chemie, GMBH.
ber of stabilized 2-norbornyl cations in superacid have also been determined.
For example, 90 and 91 have both been characterized as rapidly equilibrating
carbenium ions by lH and 13C nmr spectro~copy.~~~
The Bicyclo[2.2.2]octyl System
Since exo-2-norbornyl arenesulfonates apparently do solvolyze with anchi-
meric assistance, the less strained 2-bicyclo[2.2.2]octyl derivatives (92) are of
interest. In this system no comparison of the rates of exo and endo derivatives
- -- -- - -
Can be made, since a substituent exo to one bridge is end6 to the-other. Stereo-
ch<ii<al studies are, however, possible. If the carbonium ion (93) were formed
from chiral92 by participation of the C,-C6 bond, it would be chiral. Scheme 5
shows that attack at C, of 93 should give retained bicyclo[2.2.2.]octyl solvolysis
products (92a), and attack at C, should give chiral exo-2-bicyclo[3.2.1]octyl
116 G. A. Olah and G. Liang, J. Amer. Chem. Soc., 96, 189, 195 (1974).