Page 326 - Mechanism and Theory in Organic Chemistry
P. 326
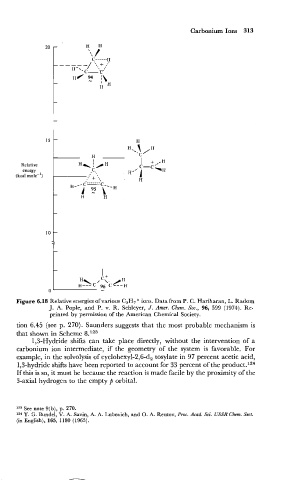
Carbonium Ions 313
Relative
energy
(kcal mole-')
Figure 6.18 Relative energies of various C3H7 + ions. Data from P. C. Hariharan, L. Radom
J. A. Pople, and P. v. R. Schleyer, J. Amer. Chem. SOG., 96, 599 (1974). Re-
printed by permission of the American Chemical Society.
tion 6.45 (see p. 270). Saunders suggests that the most probable mechanism is
that shown in Scheme 8.lZ3
1,3-Hydride shifts can take place directly, without the intervention of a
carbonium ion intermediate, if the geometry of the system is favorable. For
example, in the solvolysis of cyclohexyl-2,6-d, tosylate in 97 percent acetic acid,
1,3-hydride shifts have been reported to account for 33 percent of the pr0d~ct.l~~
If this is so, it must be because the reaction is made facile by the proximity of the
3-axial hydrogen to the empty p orbital.
lZ3 See note 9(b), p. 270.
lZ4 Y. G. Bundel, V. A. Savin, A. A. Lubovich, and 0. A. Reutov, Proc. Acad. Sci. USSR Chem. Sect.
(in English), 165, 1180 (1965).