Page 328 - Mechanism and Theory in Organic Chemistry
P. 328
Carbonium Ions 315
carbocations, 1,3-, 1,4-, 1,5- and 1,6-hydride shifts occur readily.127 "Trans-
annular shifts" were first noted when unexpected products were found128*129 in
the peroxyformic acid oxidation of medium-ring alkenes. For example, cyclo-
octene gave, in addition to minor amounts of the expected tram-1,2-diol, cyclo-
octane-cis-1,4-diol and 3- and 4-cyclooctene-01s. Either a 1,3- or 1,5-hydride
shift could bring about formation of the 1,4-diol and of the unsaturated alcohols
(see Scheme 9). That both orders of hydride shift take place in this reaction
was shown by Cope and co-workers, who treated 5,6-d2-cyclooctene oxide (102)
with 90 percent formic acid and, by determining the position of deuterium in the
products, ascertained that 1,5-migration accounted for 94 percent of the forma-
tion of 3-cycloocten- 1-01 and 6 l percent of the l ,4-dio1.130
The fact that only tram-1,2- and cis-1,4-glycols are obtained implies that
they cannot actually be formed by the simplified mechanism in Scheme 9. The
carbenium ions 99-101 should give a mixture of cis and trans glycols. However,
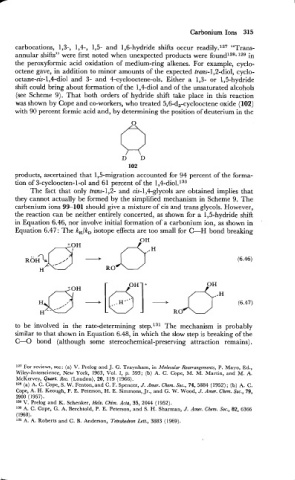
the reaction can be neither entirely concerted, as shown for a 1,5-hydride shift
in Equation 6.46, nor involve initial formation of a carbonium ion, as shown in
Equation 6.47: The k,/k, isotope effects are too small for CLH bond breaking
to be involved in the rate-determining step.131 The mechanism is probably
similar to that shown in Equation 6.48, in which the slow step is breaking of the
C-0 bond (although some stereochemical-preserving attraction remains).
12' For reviews, see: (a) V. Prelog and J. G. Traynham, in Molecular Rearrangements, P. Mayo, Ed.,
Wiley-Interscience, New York, 1963, Vol. I, p. 593; (b) A. C. Cope, M. M. Martin, and M. A.
McKervey, Quart. Rev. (London), 20, 1 19 (1966).
las (a) A. C. Cope, S. W. Fenton, and C. F. Spencer, J. Amer. Chem. Soc., 74, 5884 (1952); (b) A. C.
Cope, A. H. Keough, P. E. Peterson, H. E. Simmons, Jr., and G. W. Wood, J. Amer. Chem. Soc., 79,
3900 (1957).
.
.
lag V. Prelog and K. Schenker, Helv. Chim. Acta, 35, 2044 (1952).
laO A. C. Cope, G. A. Berchtold, P. E. Peterson, and S. H. Sharman, J. Amer. Chem. Soc., 82, 6366
(1960).
lal A. A. Roberts and C. B. Anderson, Tetrahedron Lett., 3883 (1969).