Page 324 - Mechanism and Theory in Organic Chemistry
P. 324
Carbonium Ions 31 1
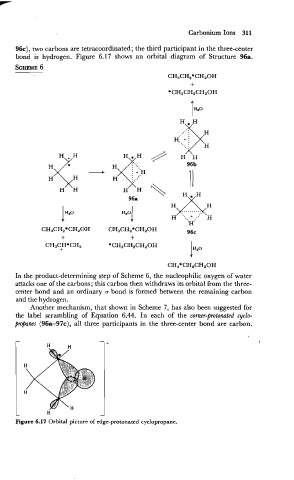
96c), two car,bons are tetracoordinated; the third participant in the three-center
bond is hydrogen. Figure 6.17 shows an orbital diagram of Structure 96a.
..........
H&H .. : H
H
'H'
In the product-determining step of Scheme 6, the nucleophilic oxygen of water
attacks one of the carbons; this carbon then withdraws its orbital from the three-
center bond and an ordinary u bond is formed between the remaining carbon
and the hydrogen.
Another mechanism, that shown in Scheme 7, has also been suggested for
the label scrambling of Equation 6.44. In each of the corner-protonated cyclo-
profianes (96a-97c), all three participants in the three-center bond are carbon.
Figure 6.17 Orbital picture of edge-protonated cyclopropane.