Page 316 - Mechanism and Theory in Organic Chemistry
P. 316
Carbonium Ions 303
sulting from participation of this bond is more highly strained than the starting
.
.
material. .Thus, according to . . ' c first ionizgs
to thehzxx&.&Comrcarrange
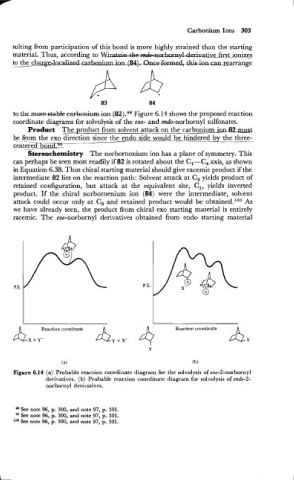
to ~~&~~buniurn Figure 6.14 shows the proposed reaction
(82).98
icrn
coordinate diagrams for solvolysis of the exo- and endo-norbornyl sulfonates.
Product The product f r p o a w
be from the exo direction since the endo side would be hindered by the three-
-
centkrtjdw
.-
Stereochemistry The norbornonium ion has a plane of symmetry. This
can perhaps be seen most readily if 82 is rotated about the C,-C, axis, as shown
in Equation 6.38. Thus chiral starting material should give racemic product if the
intermediate 82 lies on the reaction path: Solvent attack at C, yields product of
retained configuration, but attack at the equivalent site, C,, yields inverted
product. If the chiral norbornenium ion (84) were the intermediate, solvent
attack could occur only at C, and retained product would be obtained.loO As
we have already seen, the product from chiral exo starting material is entirely
racemic. The exo-norbornyl derivatives obtained from endo starting material
4
Reaction coordinate Reaction coordinate
Ax+y- + -
Figure 6.14 (a) Probable reaction coordinate diagram for the solvolysis of exo-2-norbornyl
derivatives. (b) Probable reaction coordinate diagram for solvolysis of endo-2-
norbornyl derivatives.
See note 96, p. 300, and note 97, p. 301.
See note 96, p. 300, and note 97, p. 301.
loo See note 96, p. 300, and note 97, p. 301.