Page 336 - Mechanism and Theory in Organic Chemistry
P. 336
Rearrangements to Electron-deficient Nitrogen and Oxygen 323
mediate in the Hofmann reaction has been proved, but the possibility remains
that it is at least sometimes formed.
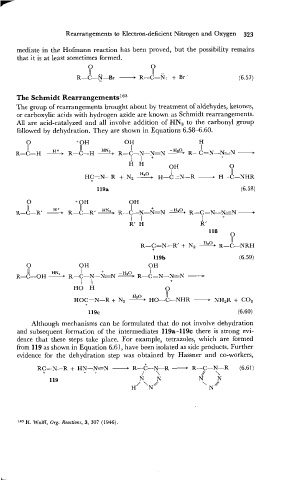
0 0
I1 .. II ..
R-C-N-Br - R-C-N: + Br-
The Schmidt Rearrangement~l~~
The group of rearrangements brought about by treatment of aldehydes, ketones,
or carboxylic acids with hydrogen azide are known as Schmidt rearrangements.
All are acid-catalyzed and all involve addition of HN, to the carbonyl group
followed by dehydration. They are shown in Equations 6.58-6.60.
0 'OH OH H
11 11 HN I R-C=N-N=N -
I
R-C-H R-C-H 3. R-C-N-N=N
l l + +
H H
OH 0
I I1
HC=N-R +, N, H-c=N-R - H-C-NHR
+
0 +OH OH
11 H+ I I
R-C-w R-C-Rg HH.- R-L-N.-&=j=--N R-c=N-N-N +
I I I +
R' H R '
118
II
E120
R-C=N-R' + Nz R-C-NRH
+
119b (6.59)
R-C=N-NEN -
R-C-OH - R-C-N-NzN -
- H20 PH
0
OH
11
I
HN,,
+
I I +
HO H 0
Hz0 11
HOCzN-R + Nz - HO-C-NHR - NHzR + COz
+
Although mechanisms can be formulated that do not involve dehydration
and subsequent formation of the intermediates 119a-119c there is strong evi-
dence that these steps take place. For example, tetrazoles, which are formed
from 119 as shown in Equation 6.61, have been isolated as side products. Further
evidence for the dehydration step was obtained by Hassner and co-workers,
le3 H. Wolff, Org. Reactions, 3, 307 (1946).