Page 345 - Mechanism and Theory in Organic Chemistry
P. 345
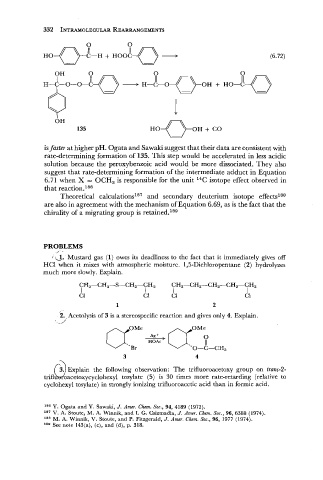
is faster at higher pH. Ogata and Sawaki suggest that their data are consistent with
rate-determining formation of 135. This step would be accelerated in less acidic
solution because the peroxybenzoic acid would be more dissociated. They also
suggest that rate-determining formation of the intermediate adduct in Equation
6.71 when X = OCH, is responsible for the unit 14C isotope effect observed in
that reaction.ls6
Theoretical calculations187 and secondary deuterium isotope effectslE8
are also in agreement with the mechanism of Equation 6.69, as is the fact that the
chirality of a migrating group is retained.lsg
PROBLEMS
/ -
J. Mustard gas (1) owes its deadliness to the fact that it immediately gives off
HCl when it mixes with atmospheric moisture. 1,5-Dichloropentane (2) hydrolyzes
much more slowly. Explain.
z., Acetolysis of 3 is a stereospecific reaction and gives only 4. Explain.
,J
3 4
3. Explain the following observation: The trifluoroacetoxy group on trans-2-
trifl 0 oacetoxycyclohexyl tosylate (5) is 30 times more rate-retarding (relative to
cyclohexyl tosylate) in strongly ionizing trifluoroacetic acid than in formic acid.
lac Y. Ogata and Y. Sawaki, J. Amer. Chem. SOC., 94, 4189 (1972).
ls7 V. A. Stoute, M. A. Winnik, and I. G. Csizrnadia, J. Amer. Chem. SOC., 96, 6388 (1974).
laa M. A. Winnik, V. Stoute, and P. Fitzgerald, J. Amer. Chetn. Soc., 96, 1977 (1974).
See
lsB note 143(a), (c), and (d), p. 318.