Page 375 - Mechanism and Theory in Organic Chemistry
P. 375
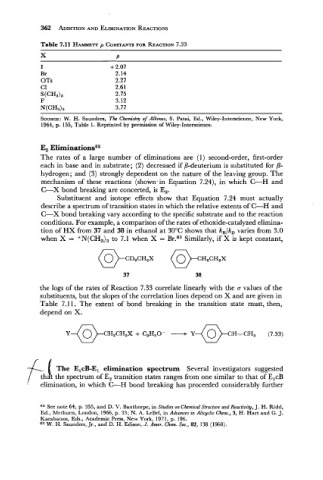
Table 7.11 HAMMEIT CONSTANTS FOR REACTION 7.33
p
I
Br
OTs
C1
SOURCE: W. H. Saunders, The Chemistry of Alkenes, S. Patai, Ed., Wiley-Interscience, New York,
1964, p. 155, Table 1. Reprinted by permission of Wiley-Interscience.
The rates of a large number of eliminations are (1) second-order, first-order
each in base and in substrate; (2) decreased if /3-deuterium is substituted for /3-
hydrogen; and (3) strongly dependent on the nature of the leaving group. The
mechanism of these reactions (shown-in Equation 7.24), in which G H and
G X bond breaking are concerted, is E,.
Substituent and isotope effects show that Equation 7.24 must actually
describe a spectrum of transition states in which the relative extents of C-H and
C-X bond breaking vary according to the specific substrate and to the reaction
conditions. For example, a comparison of the rates of ethoxide-catalyzed elimina-
tion of HX from 37 and 38 in ethanol at 30°C shows that k,/k, varies from 3.0
when X = +N(CH,), to 7.1 when X = Br.83 Similarly, if X is kept constant,
the logs of the rates of Reaction 7.33 correlate linearly with the a values of the
substituents, but the slopes of the correlation lines depend on X and are given in
Table 7.1 1. The extent of bond breaking in the transition state must, then,
depend on X.
I"
The ElcB-E, elimination spectrum Several investigators suggested
that the spectrum of E, transition states ranges from one similar to that of ElcB
elimination, in which C-H bond breaking has proceeded considerably further
82 See note 64, p. 355, and D. V. Banthorpe, in Studies on Chemical Structure and Reactivity, J. H. Ridd,
Ed., Methuen, London, 1966, p. 33; N. A. LeBel, in Advances in Alicyclic Chem., 3, H. Hart and G. J.
Karabatsos, Eds., Academic Press, New York, 197 1, p. 196.
83 W. H. Saunders, Jr., and D. H. Edison, J. Amer. Chem. Soc., 82, 138 (1960).